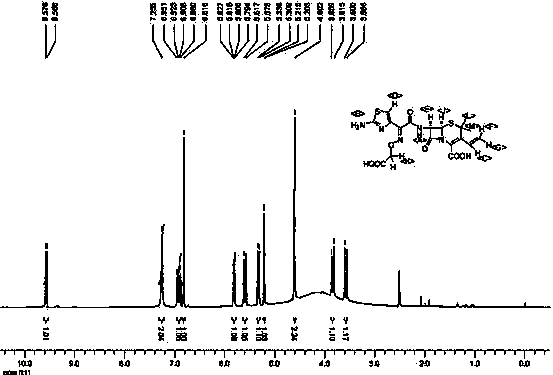
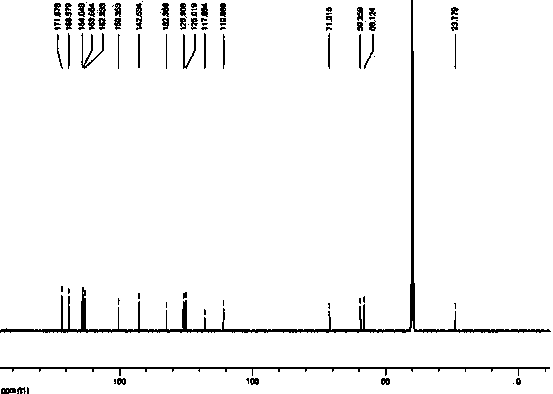
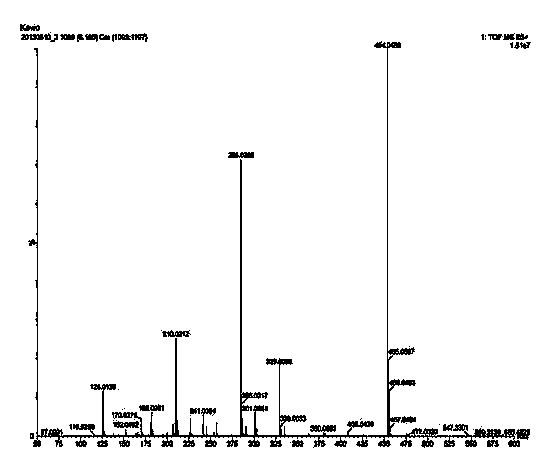
Method for preparing cefixime compound
A technology of cefixime and cefixime methyl ester, which is applied in the field of preparation of cefixime compounds, can solve the problems of harsh reaction conditions, expensive solvents, unfavorable continuity, etc., and achieve increased product yield, increased yield and Purity, the effect of shortening the production cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] A preparation method of cefixime compound, the steps are as follows:
[0033] 1) In a 1000mL four-neck flask, add 300mL of dichloromethane, 45mL of ethanol, and 15mL of purified water, stir and cool down to 0-2°C, add 50g of 7-AVCA, 100g of MICA active ester, and slowly add triethylamine dropwise at 5°C It takes about 0.5 hours for 25g of amine, and the temperature is raised to 5-10°C after the addition is completed, and the reaction is carried out under this condition for 5 hours, the pH is controlled at 7.5-8.0, and the reaction is stopped when 7-AVCA<0.5wt% in the reaction solution is detected by HPLC. A solution of cefixime methyl ester was prepared.
[0034] 2) Add 150mL of purified water to the above cefixime methyl ester solution, stir for 10 minutes, let stand to separate the water phase, extract the organic phase twice with 150mL of pure water, combine the water phase, add 1g hydrosulfite, 1g ethylenediamine Tetraacetic acid and 5g activated carbon were decolo...
Embodiment 2
[0039] A kind of preparation method step of cefixime compound is as follows:
[0040] 1) In a 1000mL four-neck bottle, add 350mL of chloroform, 60mL of methanol, and 25mL of purified water, stir and cool down to 0-5°C, add 50g of 7-AVCA, 120g of MICA active ester, and slowly add 28g of triethylamine dropwise at 5°C After about 1 hour, the dropwise addition was completed and the temperature was raised to 8-12°C. Under this condition, the reaction was carried out for 5.5 hours, and the pH was controlled at 7.5-8.0. The reaction was stopped by detecting 7-AVCA<0.5% in the reaction solution by HPLC, and Cefixime A was obtained. ester solution.
[0041] 2) Add 200 mL of purified water to the above cefixime methyl ester solution, stir for 10 minutes, and let stand to separate the water phase. The organic phase was extracted twice with 100 mL of purified water, the aqueous phase was combined, 0.5 g of hydrosulfite and 0.5 g of ethylenediaminetetraacetic acid were added, 2.5 g of act...
Embodiment 3
[0045] A preparation method of cefixime compound, the steps are as follows:
[0046] 1) In a 1000mL four-necked bottle, add 300mL of ethyl acetate, 50mL of methanol, and 30mL of purified water, stir and cool down to 5-8°C, add 50g of 7-AVCA, 125g of MICA active ester, and slowly add 25g of cyclohexylamine dropwise at 5°C After about 0.5 hours, the dropwise addition was completed and the temperature was raised to 10-15°C. Under this condition, the reaction was carried out for 6 hours, and the pH was controlled at 8.0-8.5. The 7-AVCA<0.5% in the reaction solution was detected by HPLC to stop the reaction, and Cefixime A was obtained. ester solution.
[0047] 2) After the reaction, add 150 mL of purified water, stir for 10 minutes, and let stand to separate the water phase. The organic phase was extracted twice with 150 mL of pure water, the aqueous phase was combined, 1.5 g of hydrosulfite and 1.5 g of ethylenediaminetetraacetic acid were added, 5 g of activated carbon was deco...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com