Amphiphilic molecule hydrogel based on polyamino acid
A technology of amphiphilic molecules and polyamino acids, which is applied in the field of biodegradable materials and drug controlled release, can solve the problems of difficult large-scale production, high cost, and difficulty in hydrogel synthesis, and achieve easy large-scale production and low cost. Inexpensive, good biocompatibility effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1, the preparation of polyamino acid-based amphiphile molecule and its hydrogel shown in formula II
[0035]
[0036] (1) First, add 90 ml of diethylene glycol monomethyl ether and 15 grams of glutamic acid into the round bottom flask. The ratio of glutamic acid to diethylene glycol monomethyl ether in this system is 1:6 , put the suspension in an ice-water bath, wait until it is cooled to 0°C, slowly drop into 10 ml of concentrated sulfuric acid (wherein the ratio of glutamic acid to concentrated sulfuric acid is 1:1.1 by mass), return to 20°C, react at 20°C for 48 hours. Pour the clear solution into a mixed solution of triethylamine and isopropanol (1:10 by volume), resulting in a white precipitate. Centrifuge to obtain 15.5 grams of white solid, with a yield of 61.0%;
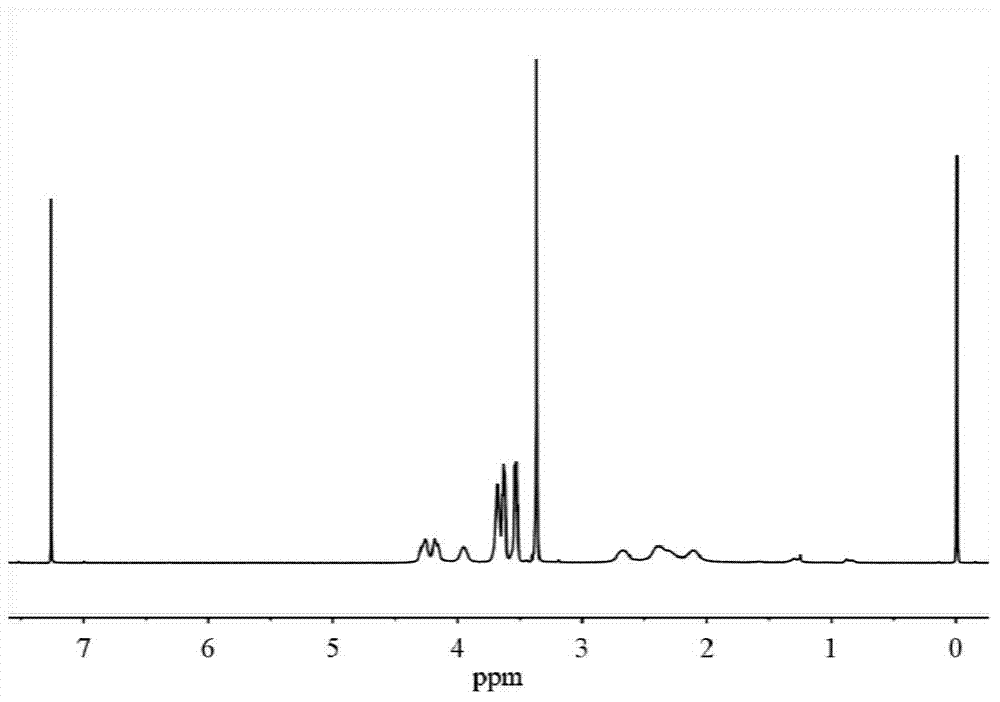
[0037] H NMR 1 H NMR (400MHz,D 2 O): δ4.31-4.22(t,2H),3.82-3.72(m,3H),3.72-3.64(m,2H),3.63-3.56(m,2H),3.39-3.32(s,3H), 2.66-2.49(m,2H),2.25-2.07(m,2H); C NMR 13 C NMR (400MHz,D 2 ...
Embodiment 2
[0045] Embodiment 2, the preparation of polyamino acid-based amphiphile molecule and its hydrogel shown in formula III
[0046]
[0047] (1) First, add 90 ml of diethylene glycol monomethyl ether and 15 grams of glutamic acid into the round bottom flask. The ratio of glutamic acid to diethylene glycol monomethyl ether in this system is 1:6 , put the suspension in an ice-water bath, wait until it is cooled to 0°C, slowly drop into 10 ml of concentrated sulfuric acid (wherein the ratio of glutamic acid to concentrated sulfuric acid is 1:1.1 by mass), return to 20°C, react at 20°C for 48 hours. Pour the clear solution into a mixed solution of triethylamine and isopropanol (1:10 by volume), resulting in a white precipitate. After centrifugation, 15.5 g of white solid was obtained with a yield of 61.0%.
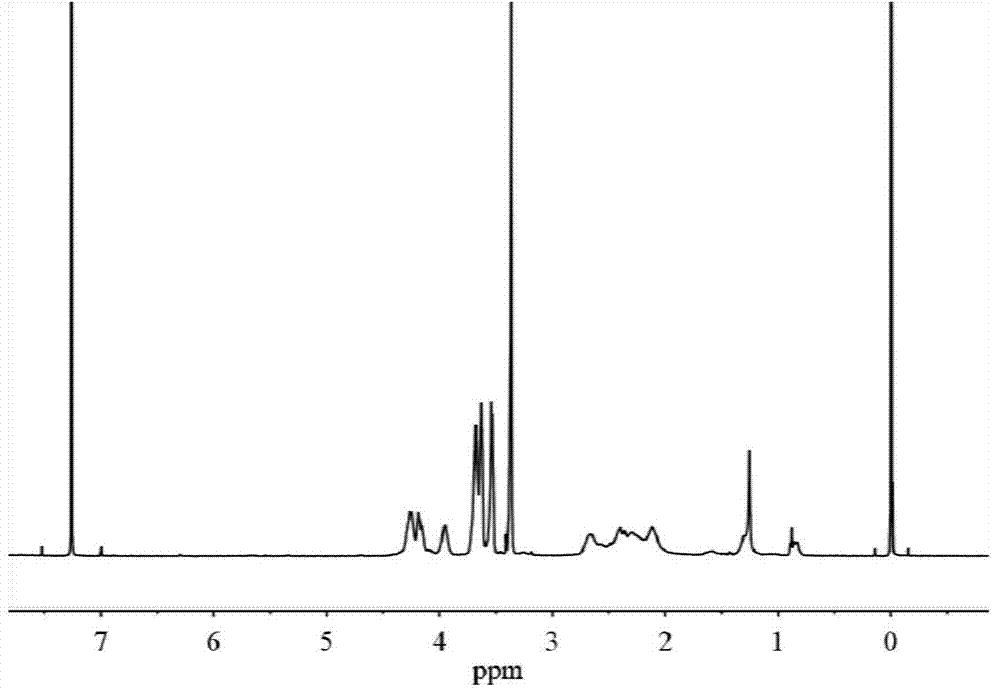
[0048] H NMR 1 H NMR (400MHz,D 2 O): δ4.31-4.22(t,2H),3.82-3.72(m,3H),3.72-3.64(m,2H),3.63-3.56(m,2H),3.39-3.32(s,3H), 2.66-2.49(m,2H),2.25-2.07(m,2H); C NMR 13 C NMR (40...
Embodiment 3
[0056] Embodiment 3, the preparation of polyamino acid-based amphiphile molecules shown in formula IV
[0057]
[0058] (1) First, add 90 ml of diethylene glycol monomethyl ether and 15 grams of glutamic acid into the round bottom flask. The ratio of glutamic acid to diethylene glycol monomethyl ether in this system is 1:6 , put the suspension in an ice-water bath, wait until it is cooled to 0°C, slowly drop into 10 ml of concentrated sulfuric acid (wherein the ratio of glutamic acid to concentrated sulfuric acid is 1:1.1 by mass), return to 20°C, react at 20°C for 48 hours. Pour the clear solution into a mixed solution of triethylamine and isopropanol (1:10 by volume), resulting in a white precipitate. After centrifugation, 15.5 g of white solid was obtained with a yield of 61.0%.
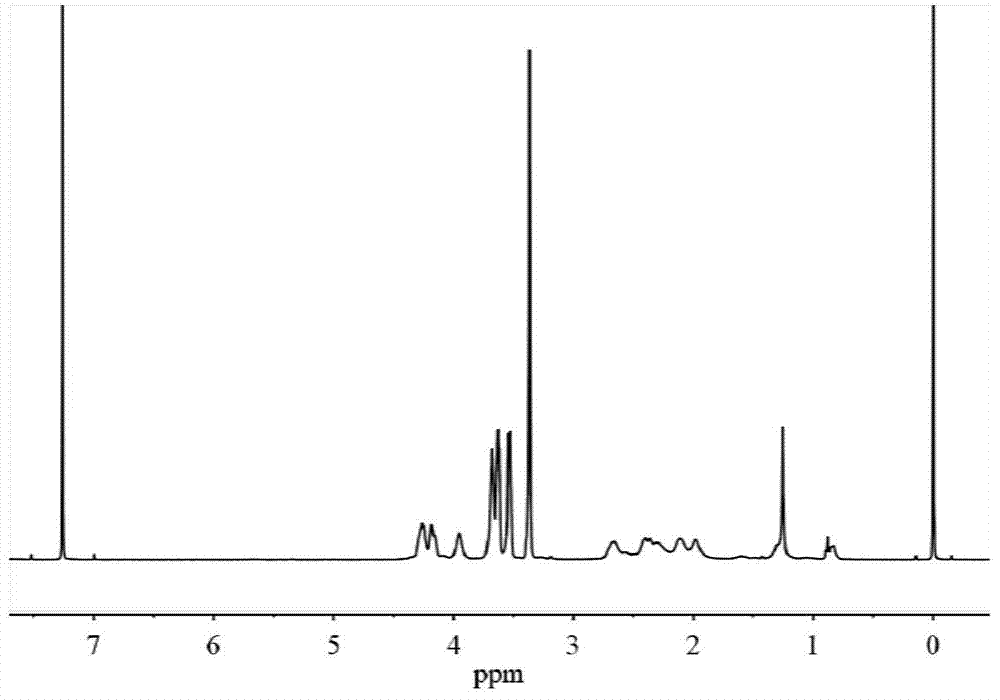
[0059] H NMR 1 H NMR (400MHz,D 2 O): δ4.31-4.22(t,2H),3.82-3.72(m,3H),3.72-3.64(m,2H),3.63-3.56(m,2H),3.39-3.32(s,3H), 2.66-2.49(m,2H),2.25-2.07(m,2H); C NMR 13 C NMR (400MHz,D 2 O): δ17...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of polymerization | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com