Mutant Taq enzyme and preparation method thereof
A mutant, nucleotide sequence technology, applied in the biological field, can solve problems such as limitations, achieve the effect of improving thermal stability and meeting the needs of industrial and research applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1: Site-directed mutagenesis of Taq enzyme plasmid
[0041] The plasmid containing the natural Taq enzyme gene was subjected to PCR amplification. The synthetic amplification primer is the mutation primer, which contains the site to be mutated and has replaced the base to be mutated. After amplification, the original master plate is digested with an enzyme, that is, the unmutated Taq gene template as the initial template is digested. The product of PCR, that is, the plasmid containing the mutated Taq enzyme gene was transformed into competent cells. The plasmid containing the mutant enzyme gene can be obtained by picking the clone.
[0042] 50μL PCR reaction system: 10×buffer (100mmol / L KCl, 100mmol / L (NH4) 2 SO 4, 200mmol / L Tris-HCl pH8.8, 20mmol / L MgSO 4 , 1% TritonX-100, 1mg / mL BSA) 5μL, 10mmol / L dNTP 0.5μL, 125ng each of mutation primers, 1U pfu enzyme, 1μL containing 50ng plasmid template, add ddH 2 0 to 50 μL. Reaction conditions: Denaturation at 95...
Embodiment 2
[0044] Example 2: Expression and purification of mutant modified Taq enzyme
[0045] Inoculate 50 μL strains in 5ml LB liquid medium and shake for 6-8h, then transfer to 250mL LB liquid medium, shake for 4-6h, add IPTG to a final concentration of 50mmol / L, and continue shaking overnight. Centrifuge 250mL of bacterial liquid in a 50mL centrifuge tube at 5000rpm for 10min to collect the bacterial cells, add 5mL of Binding buffer per 100mL of culture, add lysozyme to a final concentration of 1mg / mL, place on ice for 1h, shake at 4°C for 10min, add TritonX-100 to The final concentration is 1%, vigorously shake and mix, shake at 4°C for 10min, centrifuge at 5,000rpm for 30min, take the supernatant (reserved sample of 20μL bacterial cell crushing product), bathe in 75°C water for 1h, shake from time to time, centrifuge at 5000rpm for 30min, take the supernatant (Reserve 20 μL of crude enzyme solution). Gently mix the Ni-gel, take 1mL to the column, 1mL ddH 2 O washed twice, with 1...
Embodiment 3
[0046] Example 3 SDS-PAGE protein electrophoresis verification enzyme protein size and purity
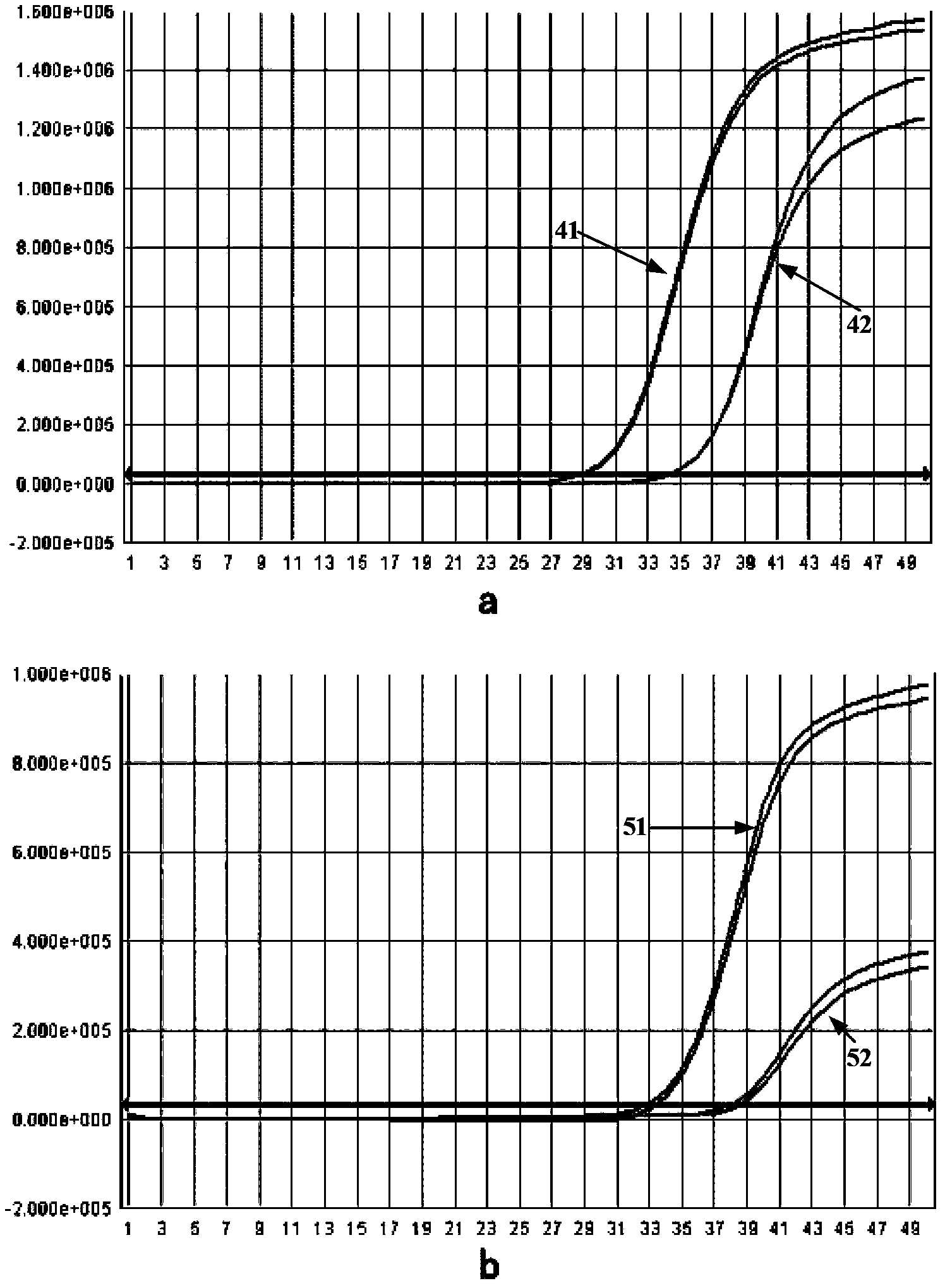
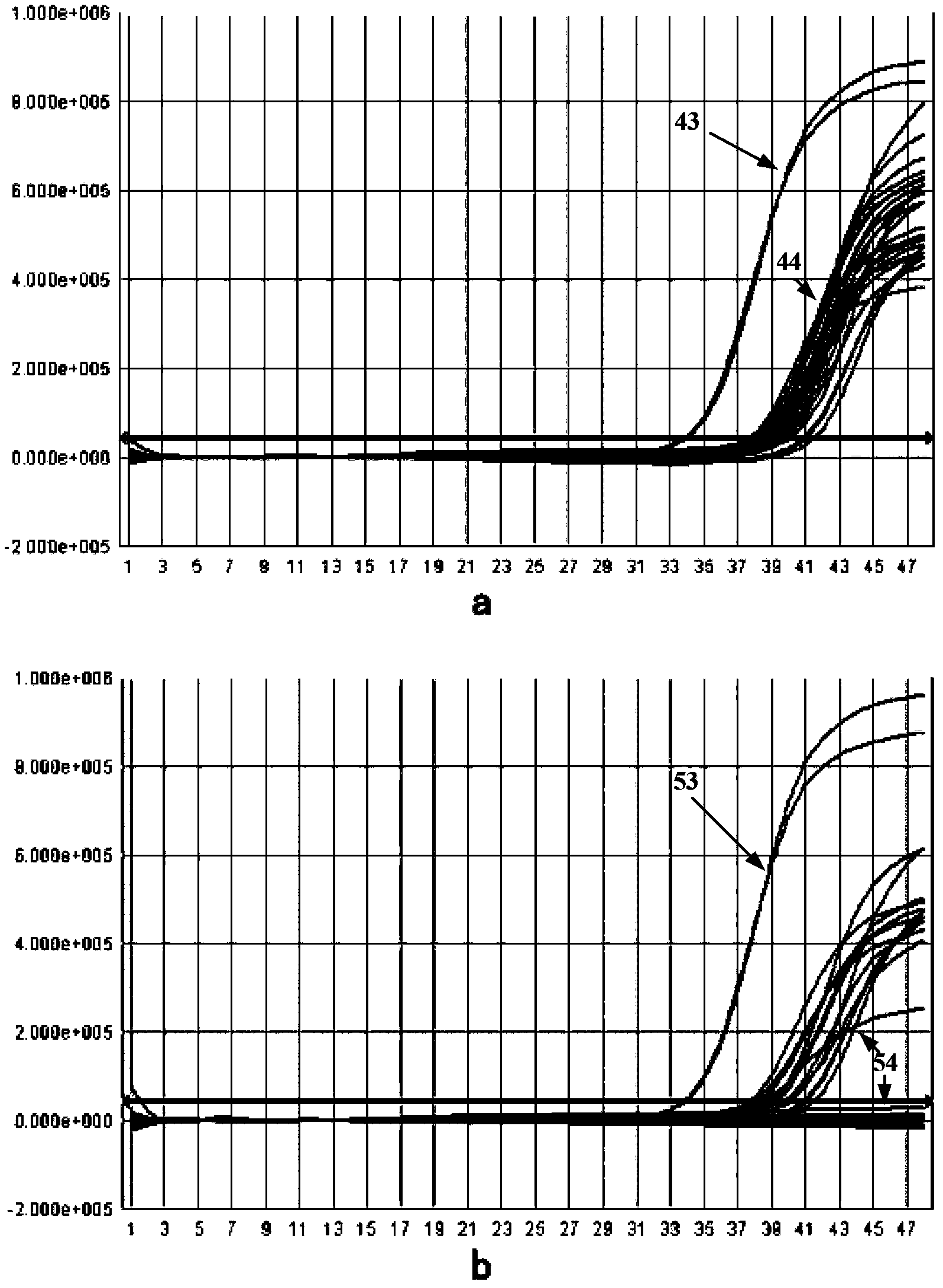
[0047] The mutant Taq enzyme and the wild-type Taq enzyme obtained above were subjected to SDS-PAGE protein electrophoresis detection to identify the size and purity of the mutant Taq enzyme protein.
[0048] Method: Take 40ul of each sample, add 10ul of 5×Loading Buffer, bathe in boiling water for 10min, and load 5ul of sample. The constant pressure of the stacking gel is 100V, and the constant pressure of the separating gel is 180V. Coomassie brilliant blue staining.
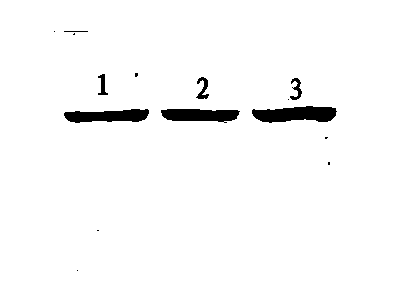
[0049] Result: Please refer to figure 1 , wherein, 1 is the wild-type Taq enzyme, 2 and 3 are mutant Taq enzymes, and each electrophoresis band is pressed into a straight line in the stacking gel; it can be seen from the figure that the mutant Taq enzyme and the wild-type Taq enzyme band The size is consistent; the background of the rubber surface is clean, the sample bands are clear, and there are no visible ban...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com