Telmisartan composition
A technology of telmisartan and composition, which is applied in the field of telmisartan pharmaceutical composition, can solve the problems of poor compressibility of granules, prolong production cycle, increase material loss and energy consumption, etc., achieve high dissolution rate and improve dissolution The effect of reducing material loss
Active Publication Date: 2014-01-22
DISHA PHARMA GRP
View PDF10 Cites 3 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
[0005] The above process is also a common process for preparing telmisartan tablets at present. Its common feature one is that telmisartan is prepared into sodium salt earlier. This process prolongs the production cycle and increases material loss and energy consumption.
The second is that the granules obtained by dissolving telmisartan in sodium hydroxide solution through spray drying or fluidized bed granulation in one step have poor compressibility. sex
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
[0022] Prescription: telmisartan 80g, sodium hydroxide 6.72g, mannitol (co-crushed) 1640g (of which, 40g was co-pulverized, used as diluent 1600g), lactose 120g, povidone K3024g, wetting agent 75% ethanol aqueous solution amount , magnesium stearate 8g, prepare 1000 tablets according to the preparation method described in the technical scheme part.
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
| Property | Measurement | Unit |
|---|---|---|
| Average hardness | aaaaa | aaaaa |
Login to View More
Abstract
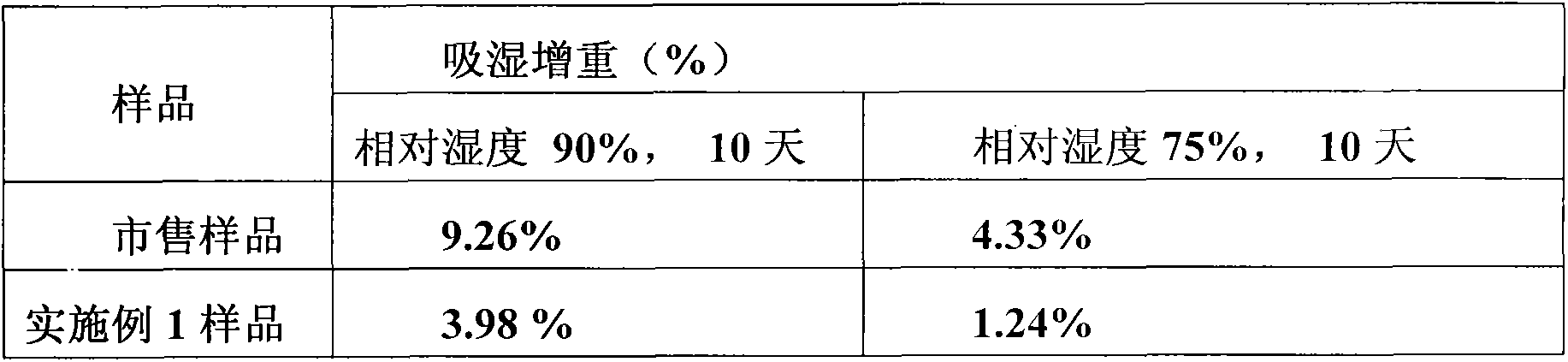
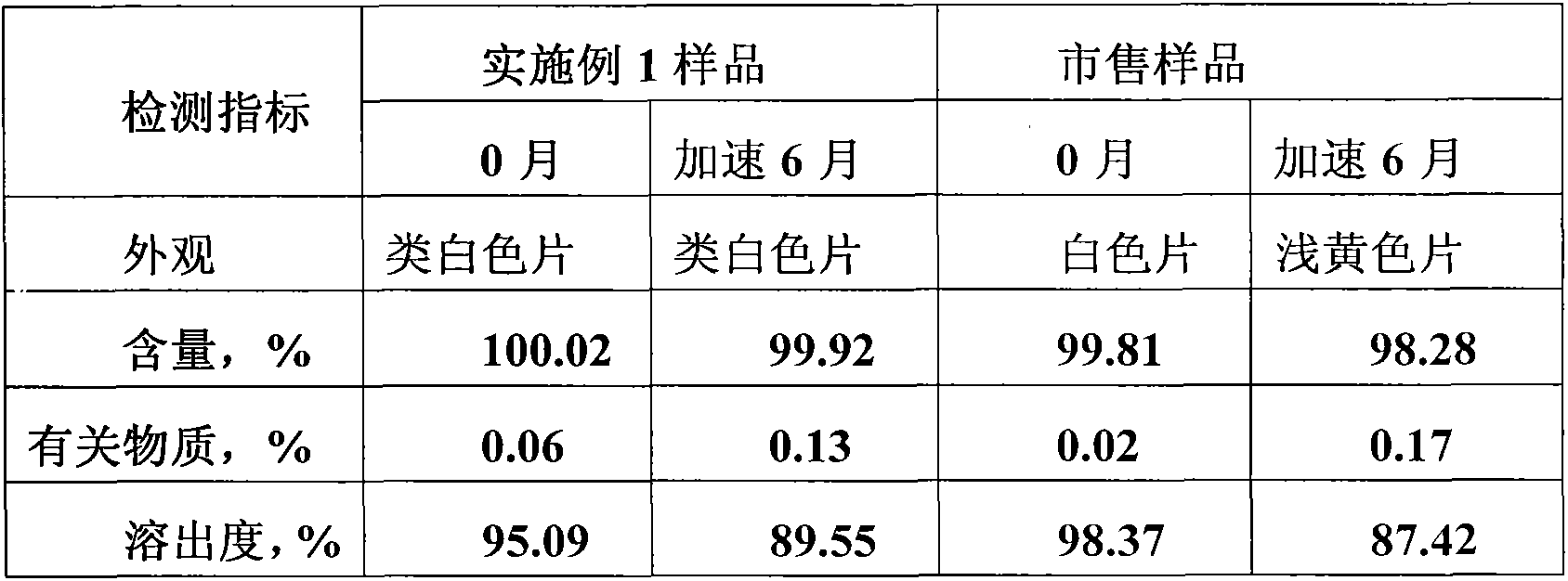
The invention relates to a telmisartan pharmaceutical composition for treating vascular hypertension, and belongs to the technical field of medicines. The technical scheme of the invention is characterized by adopting a micronization technology: a mixture of a main drug, sodium hydroxide and mannitol is micronized by an air-flow pulverizer, the D90 of the micronized mixture of telmisartan, sodium hydroxide and mannitol is between 3.0 [mu]m and 5.0 [mu]m. According to the technical scheme of the invention, a preparation method of telmisartan tablets is simplified, the preparation period is shortened, the material consumption is reduced, and moreover, the tablets are ensured to have higher dissolution rate; and secondly, through the use of the micronization technology, the drug dissolution rate is improved, the particles prepared by the micronization technology have quite good fluidity and compressibility, sorbitol having quite strong hygroscopicity is not required to be used, and the stability of the sample is improved.
Description
[0001] Technical field: the present invention relates to a kind of telmisartan pharmaceutical composition for the treatment of hypertension, which belongs to the technical field of medicine. Background of the invention: [0002] Hypertension is a common chronic disease. It appears to be an independent disease, but it is actually an important risk factor for cardiovascular, cerebrovascular and renal diseases. If not treated properly, it can lead to severe stroke, The occurrence of hypertensive complications such as myocardial infarction, heart failure or renal failure. [0003] Telmisartan is a non-peptide angiotensin II receptor antagonist with definite curative effect and few side effects, and is clinically used to treat various types of hypertension. [0004] Telmisartan is white or off-white powder at room temperature, insoluble in water. In order to increase the solubility of the tablet, the patent ZL200480011725 first prepares telmisartan into sodium salt, then sprays an...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): A61K9/20A61K31/4184A61J3/10A61P9/12
Inventor 白莉许蕾连艳菊
Owner DISHA PHARMA GRP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com