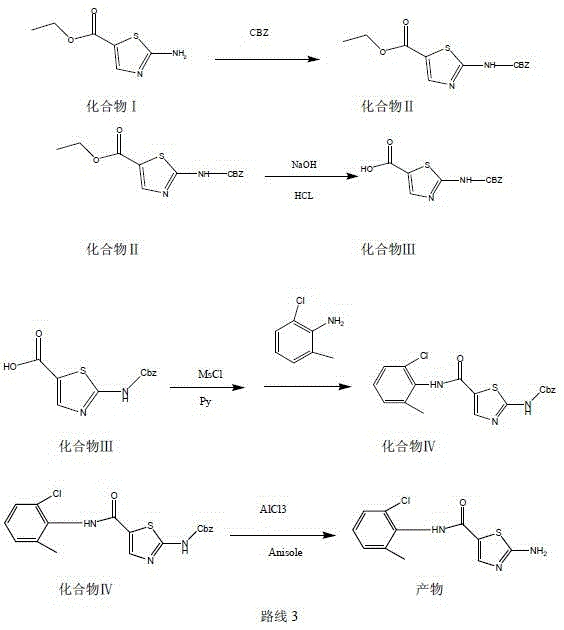
Method for synthesizing 2-amino-N-(2-chloro-6-methylphenyl)thiazole-5-carboxamide
A technology of methylphenyl and aminothiazole, applied in the field of medicine, can solve the problems of difficult raw materials, high cost, unfavorable production and the like, and achieve the effect of simplifying difficulties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Suspend 172g (1mol) of ethyl 2-aminothiazole-5-carboxylate in 1000g of dichloromethane, add 168g (2mol) of sodium bicarbonate, stir evenly, and at the same time lower the temperature to 10-15°C, and control the dropping rate at 3 Within 1 hour, 255g (1.5mol) of benzyl chloroformate was dropped into the mixture, and bubbles appeared during the dropwise addition. After the dropwise addition, it was raised to room temperature and reacted for 5 hours. Dichloromethane was recovered under reduced pressure in a water bath at 40°C. After evaporating to dryness, add 2000g of purified water for beating for 30 minutes, filter and wash with water, put the filter cake into 1000g of methanol, add 100g of glacial acetic acid, beat for 30 minutes at room temperature, lower to 0-5°C and stir for 1 hour, and suction filter to obtain an off-white solid. Air-dried at 50°C to obtain 275 g of compound II, with a yield of 90%.
Embodiment 2
[0029] Put 100g of sodium hydroxide into 1500g of 30% ethanol aqueous solution, and control the temperature below 30°C. Stir to dissolve until clear, and drop 308 g (1 mol) of the product of Example 1 at this temperature. Incubate at 30°C for 8 hours. Cool down to 0-5°C, add 2N concentrated hydrochloric acid dropwise to adjust the pH to 3, a large amount of off-white solid precipitates, stir at 0-5°C for 1 hour, then filter with suction, wash the filter cake with 1000g of purified water, and dry it with air at 80°C to obtain the compound Ⅲ dry product 260g, yield 93%.
Embodiment 3
[0031] Put 280g (1mol) of the product of Example 2 into 1000g of pyridine, lower the temperature to 0-5°C, add 125g (1.1mol) of methanesulfonyl chloride dropwise within 1 hour, keep the temperature for 5 hours after the drop, and then Add 156 g (1.1 mol) of 2-chloro-6-methylaniline and slowly raise the temperature to 80° C., keep the reaction at this temperature for 8 hours, recover pyridine under reduced pressure, and evaporate to nearly dryness. Add 2000 g of 50% methanol water and beat at room temperature for 3 hours, filter with suction, and air-dry at 80°C to obtain 350 g of compound IV as a dry product, with a yield of 85%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com