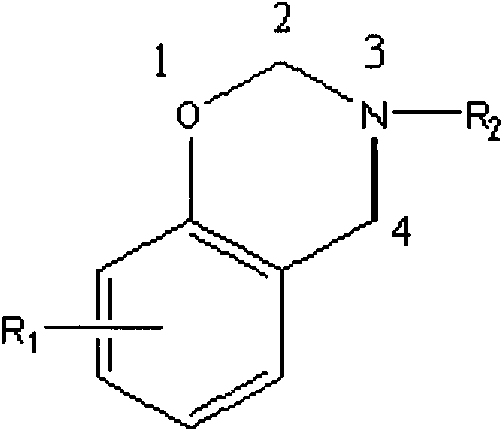
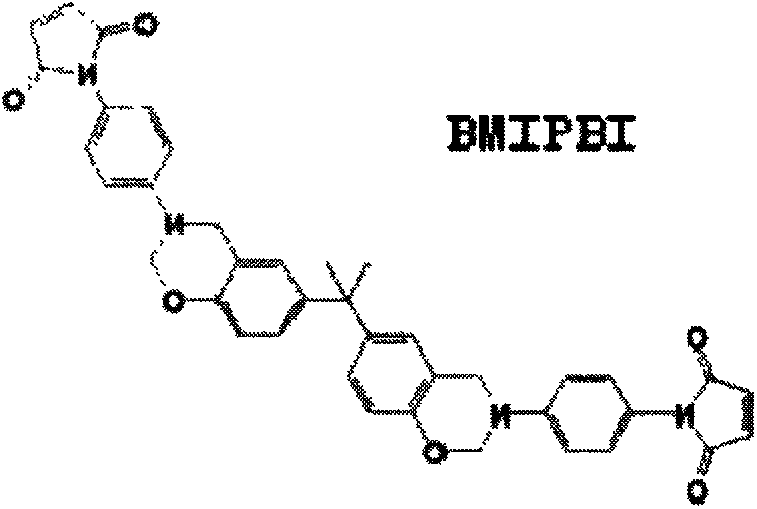
Preparation method of benzoxazine containing maleimide bisphenol A-type structure
A technology of maleimide bisphenol and benzoxazine, which is applied in the field of preparation of benzoxazine intermediates, can solve the problems of inconvenient large-scale synthesis, environmental pollution, and complicated recycling process, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] In this embodiment, the preparation method of the benzoxazine intermediate containing the maleimide bisphenol A type structure, its process steps are as follows:
[0034] (1) Synthesis reaction 1
[0035] Add 14g of formaldehyde aqueous solution, 17g of APMI, 800mL of ethyl acetate, and 100mL of water into a 1000mL round-bottomed flask in sequence, and react at room temperature for about 10h, then pour the reaction solution into a beaker filled with 1000mL of water to precipitate, vacuum filter, Washed with tap water, put into an oven and dried at 60° C. for 4 hours to obtain TMIPT as light yellow solid powder with a yield of about 96%.
[0036] (2) Synthesis reaction 2
[0037] Add 3.0g of paraformaldehyde, 20g of TMIPT, and 15.0g of bisphenol A into a 250mL round-bottomed flask in turn, raise the temperature to 160°C and react for 7h under mechanical stirring to obtain a brown solid product, which is divided into 3 times with 600mL of ethyl acetate Dissolved, then w...
Embodiment 2
[0039] In this embodiment, the preparation method of the benzoxazine intermediate containing the maleimide bisphenol A type structure, its process steps are as follows:
[0040] (1) Synthesis reaction 1
[0041] Add 21g of formaldehyde aqueous solution, 25.5g of APMI, 1200mL of ethyl acetate, and 150mL of water into a 1500mL round-bottomed flask in sequence, and react at room temperature for about 13h, then pour the reaction solution into a beaker filled with 2000mL of water to precipitate, vacuum pump filtered, washed with tap water, and dried in an oven at 60° C. for 6 h to obtain TMIPT as a light yellow solid powder with a yield of about 92%.
[0042] (2) Synthesis reaction 2
[0043] Add 4.5g of paraformaldehyde, 30g of TMIPT, and 22.5g of bisphenol A into a 250mL round-bottomed flask in turn, raise the temperature to 160°C and react for 9h under mechanical stirring to obtain a brown solid product, which is divided into 3 times with 900mL of ethyl acetate Dissolved, then...
Embodiment 3
[0045] In this embodiment, the preparation method of the benzoxazine intermediate containing the maleimide bisphenol A type structure, its process steps are as follows:
[0046] (1) Synthesis reaction 1
[0047]Add 22.4g of formaldehyde aqueous solution, 27.2g of APMI, 1280mL of ethyl acetate, and 160mL of water into a 1500mL round-bottomed flask in sequence, and react at room temperature for about 14 hours, then pour the reaction solution into a beaker filled with 1500mL of water to precipitate, vacuum Suction filtration, washing with tap water, drying in an oven at 60° C. for 4 hours to obtain TMIPT as light yellow solid powder with a yield of about 94%.
[0048] (2) Synthesis reaction 2
[0049] Add 4.8g of paraformaldehyde, 32g of TMIPT, and 24g of bisphenol A into a 250mL round-bottomed flask in turn, raise the temperature to 160°C and react for 10h under mechanical stirring to obtain a brown solid product, which is dissolved in 960mL of ethyl acetate three times, Then ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com