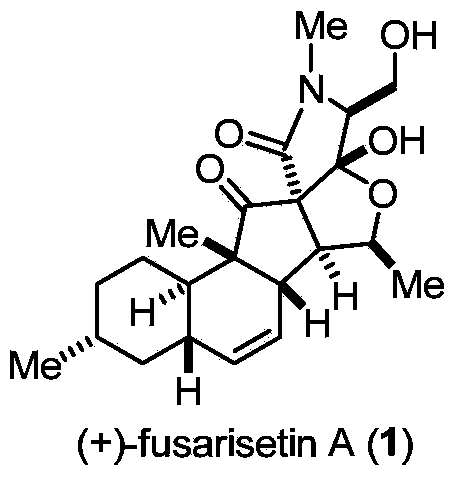
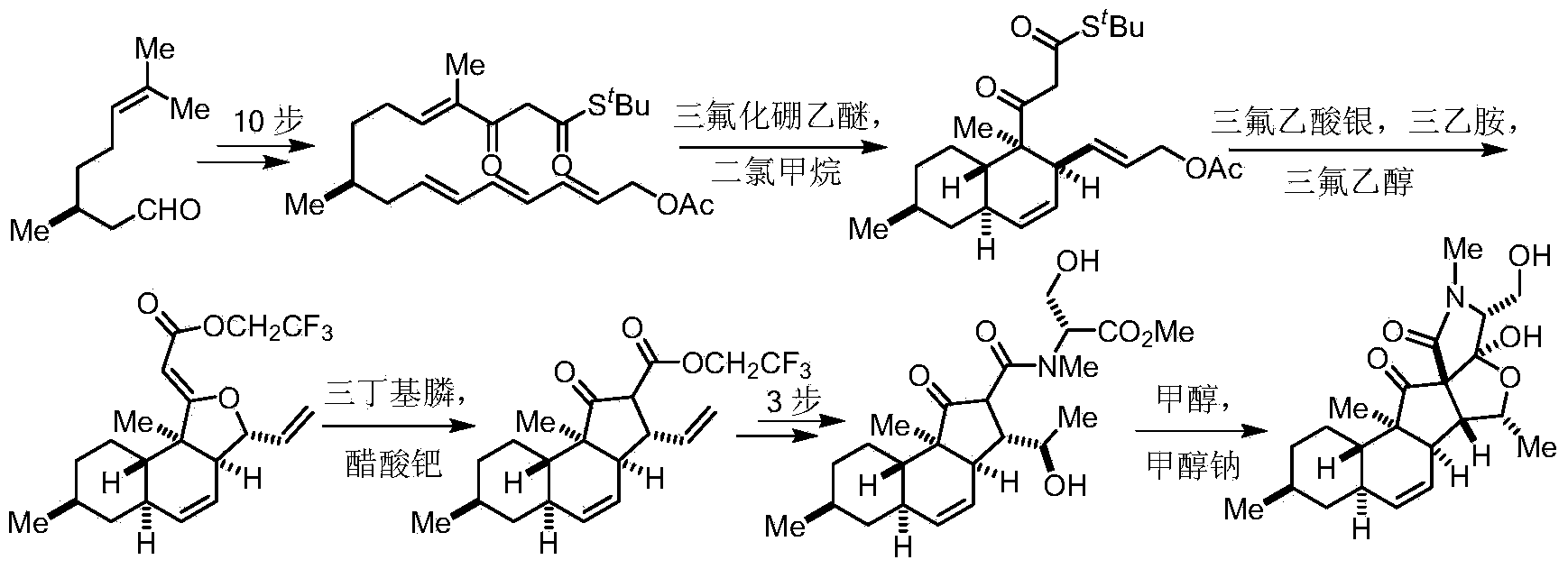
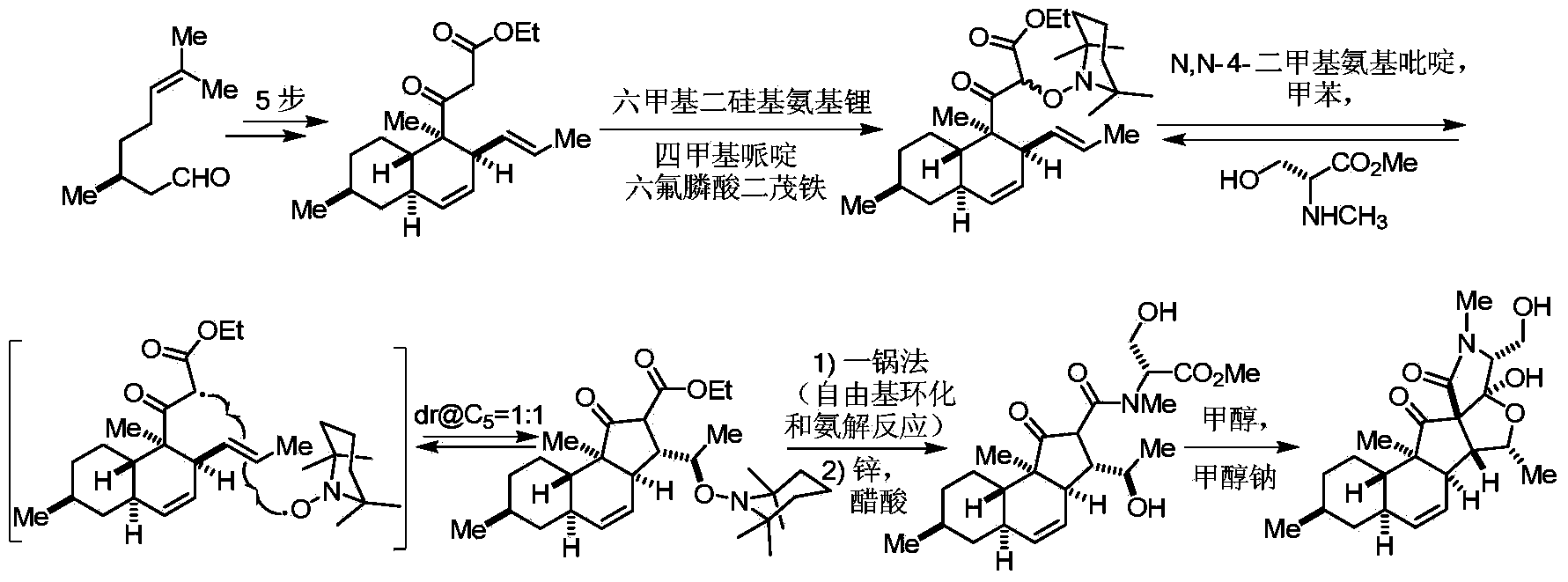
Two-type 3-acyl-2, 4-pyrrolidine-diketone compound and synthesis method thereof
A compound, pyrrolidine technology, applied in the field of new organic compounds and biomimetic synthetic routes, can solve the problems of inefficient and large-scale synthesis, excessively long routes, long routes, etc., and achieve high yield, short routes and mild reaction conditions. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Synthesis of Compound 4:
[0040]
[0041] Weigh compound 2 (45mg, 0.12mmol) in a 25mL dry reaction flask, add 12mL (0.01M) of acetic acid, and add manganese acetate (6.3mg, 0.024mmol), and react at 25°C for 5h. After the reaction is complete, the acetic acid After pumping to dryness, add 10 mL of ethyl acetate, pass through diatomaceous earth, wash the organic phase with saturated sodium chloride (5 mL×2), dry over anhydrous sodium sulfate, and spin dry. Flash column chromatography (2% methanol / dichloromethane) gave a white solid that is compound 4 (43mg, yield 88%, dr=1.1:1.0 * ), R f =0.23 (30% ethyl acetate-petroleum ether). ESI[M+Na] + calcd for [C 23 h 33 NO 6 Na] + 442.22; found 442.17. 1 HNMR (400MHz, CDCl 3 )δ5.79(4,major,d,J=10.2Hz,1H),5.76 * (epi-4,minor,d,J=10.2Hz,1H),5.58(d,J=17.9,10.2Hz,1H),5.54 * (d,J=17.9,10.2Hz,1H)4.76(s,1H),4.62 * (s,1H),4.55–4.42(m,1H),4.41–4.32(m,1H),4.41–4.32 * (m,1H),4.34-4.31 * (m,1H),4.11* (s,1H),4.01(s,1H),3.06 ...
Embodiment 2
[0043] Synthesis of compound 5:
[0044]
[0045] Weigh compound 4 (9mg, 0.024mmol) in a 10mL dry reaction tube, add 2.4mL (0.01M) of acetonitrile, and add trimethyl phosphite (85μL, 0.71mmol), react at 80°C for 2h, the reaction is complete The solvent is then spin-dried. Thin-layer chromatography (developing solvent 30% ethyl acetate / petroleum ether) gave a white solid that is compound 5 (4.1 mg, yield 48%), R f =0.11 (30% ethyl acetate-petroleum ether). ESI[M+H] + calcd for [C 23 h 33 NO 5 H] + 404.24; found 404.22. 1 HNMR (400MHz, CDCl 3 )δ5.72(ddd,J=10.0,4.6,2.7Hz,1H),5.53(d,J=10.0Hz,1H),4.52–4.17(m,3H),3.61(s,1H),3.55(d ,J=2.7Hz,1H),2.96(s,3H),2.95–2.91(m,1H),2.56(dd,J=11.1,4.6Hz,1H),1.84(t,J=14.1Hz,2H) ,1.73(d,J=12.4Hz,2H),1.65–1.51(m,2H),1.44(d,J=6.5Hz,3H),1.38(d,J=6.9Hz,3H),1.16–1.02( m,2H),0.99(d,J=6.9Hz,3H),0.91(t,J=10.6Hz,3H). 13 C NMR (100MHz, CDCl 3 )δ 215.6, 168.2, 133.4, 125.8, 102.6, 76.0, 70.3, 63.7, 63.0, 52.7, 44.8, 43.5, 41.5, 38.1, 36.9, 35...
Embodiment 3
[0047] Synthesis of compound 6:
[0048]
[0049] Weigh compound 3 (31mg, 0.069mmol) in a 20mL dry reaction flask, add 7mL (0.01M) of acetonitrile, and simultaneously add methylene blue (4.4mg, 0.014mmol) and triethylamine (38μL, 0.28mmol), at 30°C After the reaction was complete, the acetonitrile was spin-dried, and 10 mL of ethyl acetate was added, passed through diatomaceous earth, and the organic phase was washed with saturated sodium chloride (5 mL×2), dried over anhydrous sodium sulfate, and spin-dried. Separation by thin layer chromatography (developer 30% ethyl acetate / petroleum ether) gave a light blue solid compound 6 (12 mg, yield 53%, dr=2.0:1.0 * ), R f =0.23 (40% ethyl acetate-petroleum ether). ESI[M-H] - calcdfor[C 28 h 34 NO 6 ] - 480.25; found 480.29. 1 H NMR (400MHz, CDCl 3 )δ7.11 (6, major; epi-6 * ,minor,d,J=8.3Hz,2H),6.76(d,J=8.3Hz,2H),5.84–5.70(m,1H),5.55(dd,J=18.2,10.2Hz,1H),4.44( tt,J=7.2,3.5Hz,1H),3.35(t,J=7.2Hz,1H),3.27–3.12(m,2H),2.83–2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com