Ester derivatives of multi-substituted 4-methylamino-benzamidine as well as preparation method and application of ester derivatives
An aminomethyl, methyl group technology, applied in the field of chemical pharmacy, can solve the problems of large gastrointestinal side effects, increased bleeding risk, low bioavailability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
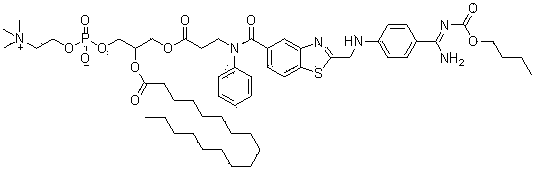
[0097] 3-{1-Methyl-2-[N-(4-(N-pentyloxycarbonyl)amidinophenyl)-aminomethyl]-benzimidazol-5-yl-carboxylic acid}-( Synthesis and preparation of N-2-pyridyl)amido-propionic acid-2-(n-heptadecylcarbonyloxy)glycerophosphorylcholine-3-yl-ester (compound 1)
[0098]
[0099] (1) Synthesis of 3-(N-2-pyridylamino)-propionic acid ethyl ester
[0100]
[0101] Under the protection of nitrogen, add ethyl acrylate (27.5g, 0.275mol) to 2-aminopyridine (22.5g, 0.25mol), stir and reflux at a temperature higher than 100°C for 24h, filter out the precipitate, concentrate the residue and use a silica gel column Column purification gave 3-(N-2-pyridylamino)-propionic acid ethyl ester (34.9 g, yield 72%) as a white solid. Mass Spectrum (ESI-MS): 195.1 (M+H) + , 217.3 (M+Na) + ;C 10 h 14 N 2 o 2 (194).
[0102] (2) Synthesis of 4-cyanoanilino-acetic acid
[0103]
[0104] Add 150ml of water to 4-cyanoaniline (6.0g, 0.05mol) and 1-chloroacetic acid (10g, 0.11mol), heat to reflux un...
Embodiment 2
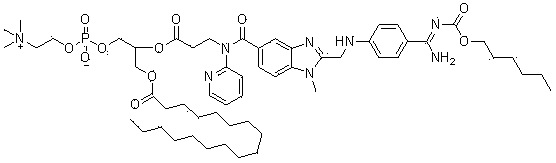
[0134] 3-{1-Methyl-2-[N-(4-(N-Hexyloxycarbonyl)amidinophenyl)-aminomethyl]-benzimidazol-5-yl-carboxylic acid}-(N Synthesis and preparation of -phenyl)amido-propionic acid-3-(n-octadecylcarbonyloxy)glycerophosphorylcholine-2-yl-ester (compound 2)
[0135]
[0136] (1) Synthesis of 3-(N-phenylamino)-ethyl propionate
[0137]
[0138] Under the protection of nitrogen, add ethyl acrylate (27.5g, 0.275mol) to aniline (23.3g, 0.25mol), add 10ml of absolute ethanol and 10ml of triethylamine, stir and reflux at a temperature higher than 100°C for 24h, filter out the precipitate The residue was concentrated and purified with a silica gel column to obtain light red solid 3-(N-phenylamino)-propionic acid ethyl ester (36.7g, yield 76%). Mass Spectrum (ESI-MS): 194.1 (M+H) + , 216.3 (M+Na) + ;C 11 h 15 NO 2 (193).
[0139] (2) Synthesis of ethyl 3-(2-nitro-1-methylamino-phenyl-4-yl)-carboxylic acid-(N-phenyl)amidopropionate
[0140]
[0141] 3-(N-Phenylamino)-propionic aci...
Embodiment 3
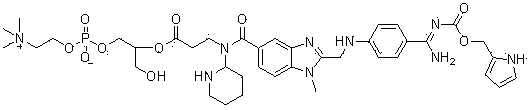
[0162] 3-{2-[N-(4-(N-Butoxycarbonyl)amidinophenyl)-aminomethyl]-benzothiazol-5-yl-carboxylic acid-(N-phenyl)}-amide Synthesis and preparation of -propionic acid-2-(n-hexadecylcarbonyloxy)glycerophosphorylcholine-3-yl-ester (compound 3)
[0163]
[0164] (1) Synthesis of 4-fluoro-3-methoxyacetamido-ethyl benzoate
[0165]
[0166] 17.4g (94.9mmol) 3-amino-4-fluoro-benzoic acid ethyl ester (refer to L.S.Fosdick, A.F.Dodds, J.Amer Chem.Soc.65,2305 (1943)) and 9.67ml (11.47g, 105.4mmol ) A solution of methoxyacetyl chloride in 310 ml of chlorobenzene was stirred at 50°C for 2 hours and then refluxed for 30 minutes. Concentrate under reduced pressure and evaporate to dryness, and purify by silica gel column chromatography (dichloromethane / ethanol=100:1) to obtain the oily product 3-amino-4-fluoro-ethyl benzoate, which solidifies into a solid after a few days (20.1g, yield rate of 83%). R f Value: 0.38 (silica gel: dichloromethane / ethanol = 19:1). Mass Spectrum (ESI-MS): ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com