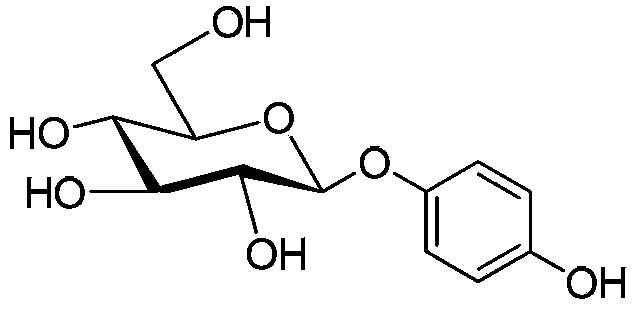
Improved beta-arbutin preparation method
A technology of arbutin and high boiling point, which is applied in the preparation of sugar derivatives, chemical instruments and methods, organic chemistry, etc., can solve the problems of increasing raw material consumption and production costs, reduce the treatment of three wastes, increase the yield, and save consumption Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0022] The mixture of 30 grams of glucose, 100 milliliters of acetic anhydride, 5 grams of sodium acetate and 100 milliliters of dimethylformamide was reacted at 100 ° C for 3 hours under stirring conditions, and the reaction solution was cooled to room temperature, and 100 milliliters of toluene was added, and mixed well , standing for layering, extracting the dimethylformamide layer with 100 milliliters of toluene again, combining the toluene layers, washing the toluene layer with 100 milliliters of water, after azeotropic dehydration, adding 15 grams of phenol p-glycolate, and reacting Liquid temperature is cooled to 5 ℃, and 50 milliliters of boron trifluoride diethyl ethers are added dropwise, and after normal temperature reaction for 4 hours, 200 milliliters of ice waters are added to the reaction liquid, after layering, the aqueous phase is extracted twice with 400 milliliters of toluene again, and the combined The toluene layer was washed with water, concentrated, and r...
example 2
[0024]The mixture of 30 grams of glucose, 100 milliliters of acetic anhydride, 5 grams of sodium acetate and 100 milliliters of dimethyl sulfoxide was reacted at 110 ° C for 2 hours under stirring conditions, and the reaction solution was cooled to room temperature, extracted three times with 300 milliliters of toluene, Combine the toluene layers, wash the toluene layer with 100 ml of water, after azeotropic dehydration, add 15 grams of phenol p-glycolate, cool the temperature of the reaction solution to 5 ° C, add dropwise 50 ml of boron trifluoride ether, and then react at room temperature After 4 hours, 200 milliliters of ice water was added to the reaction solution, and the water phase was extracted twice with 400 milliliters of toluene after the layers were separated. The combined toluene layers were washed with water, concentrated, and recrystallized with methanol to obtain 63 grams of needle-like crystals. Calculate, total recovery is 79%. Recording crystal fusing point...
example 3
[0026] The mixture of 10 grams of glucose, 35 milliliters of acetic anhydride, 2 grams of sodium acetate and 50 milliliters of dimethylformamide was reacted at 100° C. for 3 hours under stirring conditions, and the reaction solution was cooled to room temperature, extracted three times with 150 milliliters of toluene, The toluene layers were combined, and the dimethylformamide layer was azeotropically distilled with toluene to remove acetic acid, then added 10 grams of glucose and 26 milliliters of acetic anhydride, and reacted at 100° C. for 3 hours under stirring conditions. After the reaction solution was cooled to room temperature, Extract three times with 150 ml of toluene, and combine the toluene. After repeating this operation five times, all the toluene layers were combined to about 750 ml. Wash with 300 milliliters of water, after azeotropic dehydration treatment, add 25 grams of phenol p-hydroxyacetate, cool the temperature of the reaction solution to 5° C., add drop...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com