Three-dimensional copper nanowire array current collector for lithium ion battery and production method of three-dimensional copper nanowire array current collector
A technology of lithium-ion batteries and copper nanowires, applied in electrode manufacturing, battery electrodes, secondary batteries, etc., can solve problems such as poor adhesion between copper foil current collectors and active materials, poor electrochemical cycle performance, and complicated preparation procedures , to achieve good electrochemical performance, relieve volume change and stress, and shorten the effect of diffusion path
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
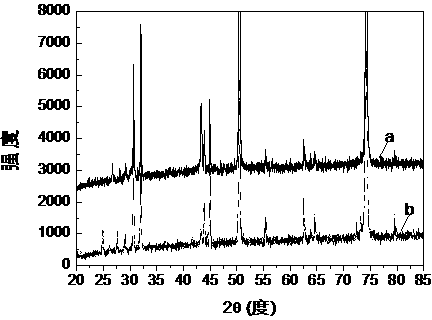
Image
Examples
Embodiment 1
[0035] 1. Soak a 1.5×1.5 cm copper strip in 0.1M hydrochloric acid solution for 3 minutes to remove oxides on the copper surface, then wash it with distilled water for 5 times, then wash it with ethanol for 3 times, and dry it for later use; configure the concentration of 2M KOH solution or NaOH solution is ready for use.
[0036] 2. Take the red copper strip as the anode, the 304 stainless steel sheet as the cathode, the saturated mercurous chloride electrode as the reference electrode, the distance between the anode and the cathode is about 3cm, and 2M KOH solution or NaOH solution as the electrolyte. Inert gas (high purity N 2 or Ar 2 etc.) about 30 min in addition to O 2 , and then controlled a constant current density of 2.5 mA / cm 2 , the anodic oxidation time is 12 min, and blue Cu(OH) is formed on the surface of the copper strip 2 The film was washed 3 times with distilled water and dried for later use.
[0037] 3. Generate blue Cu(OH) on the surface 2 The copper ...
Embodiment 2
[0044] 1. Soak a 1.5×1.5 cm copper strip in 1M hydrochloric acid solution for 5 minutes to remove oxides on the copper surface, then wash it with distilled water for 5 times, then wash it with ethanol for 3 times, and dry it for later use; prepare KOH with a concentration of 1M solution or NaOH solution for later use.
[0045] 2. Take the red copper strip as the anode, the 304 stainless steel sheet as the cathode, and the saturated mercurous chloride electrode as the reference electrode. The distance between the anode and the cathode is about 3cm. 1M KOH solution or NaOH solution is the electrolyte. Inert gas (high purity N 2 or Ar 2 etc.) about 30 min in addition to O 2 , and then controlled a constant current density of 2.5 mA / cm 2 , the anodic oxidation time is 20min, and blue Cu(OH) is formed on the surface of the copper strip 2 The film was washed 3 times with distilled water and dried for later use.
[0046] 3. Generate blue Cu(OH) on the surface 2 The copper strip...
Embodiment 3
[0053] 1. Soak a 1.5×1.5 cm copper strip in 2M hydrochloric acid solution for 4 minutes to remove oxides on the copper surface, then wash it with distilled water for 5 times, then wash it with ethanol for 3 times, and dry it for later use; configure the concentration of 0.1M KOH solution or NaOH solution is ready for use.
[0054] 2. Take the red copper strip as the anode, the 304 stainless steel sheet as the cathode, the saturated mercurous chloride electrode as the reference electrode, the distance between the anode and the cathode is about 3cm, and 0.1M KOH solution or NaOH solution as the electrolyte. Inert gas (high purity N 2 or Ar 2 etc.) about 30 min in addition to O 2 , and then controlled a constant current density of 0.5 mA / cm 2 , the anodic oxidation time is 30 min, and blue Cu(OH) is formed on the surface of the copper strip 2 The film was washed 3 times with distilled water and dried for later use.
[0055] 3. Generate blue Cu(OH) on the surface 2 The coppe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com