Synthesis method of saxagliptin chiral intermediate
A sequence and carrier technology, applied in the field of biopharmaceuticals, can solve problems such as difficult to increase production capacity, decreased thermal stability, and high energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] The enzyme activity assay of embodiment 1PDH and FDH
[0038] The enzyme activity of PDH was measured at 30°C, and the reaction system contained 0.4mM NADH, 50mM keto acid A1 (dissolved in 1 equivalent of NaOH), and 0.75M ammonia water (adjusted to pH 8.75 with HCl). After adding phenylalanine dehydrogenase, the change of the ultraviolet absorption value of the reaction system was detected at 340 nm.
[0039] The enzyme activity of FDH was measured at 30°C. The reaction system contained 1mM NAD, 100mM ammonium formate, and 100mM potassium phosphate (pH8.0) buffer solution. After adding formate dehydrogenase, the change of UV absorption value at 340nm was detected within 30 minutes. .
[0040] Enzyme activity definition:
[0041] Every 0.1 change in the UV absorbance value at 340nm corresponds to a 0.016mM change in the concentration of NADH. Therefore, the enzyme activity calculation formula is obtained:
[0042] U = A ...
Embodiment 2
[0046] Embodiment 2 substrate and product analysis method
[0047] The product was detected by high-performance liquid chromatography (HPLC): water and acetonitrile (45:55) were used as the mobile phase, the chromatographic column was ODS-18 reversed-phase column, Shimadzu LC-15C high-performance liquid chromatography, and ultraviolet absorption was detected at 210nm ; The reaction system was diluted with water and acetonitrile (45:55), centrifuged and filtered with a nylon membrane, then injected for detection, the retention time of the keto acid substrate 2-(3-hydroxy-1-adamantyl)-2-glyoxylic acid The peak retention time of the amino acid product was 4.19 minutes, and the retention time of the Boc-protected amino acid was 2.1 minutes.
[0048] Optical purity e.e. values were analyzed on Agilent 1260 series HPLC using AD-H chiral column (Diacel Chemical). The retention time of the R-configuration product was 3.5 min, and the retention time of the S-configuration product was...
Embodiment 3
[0050] The cloning of embodiment 3PDH and construction E.coli mutant library
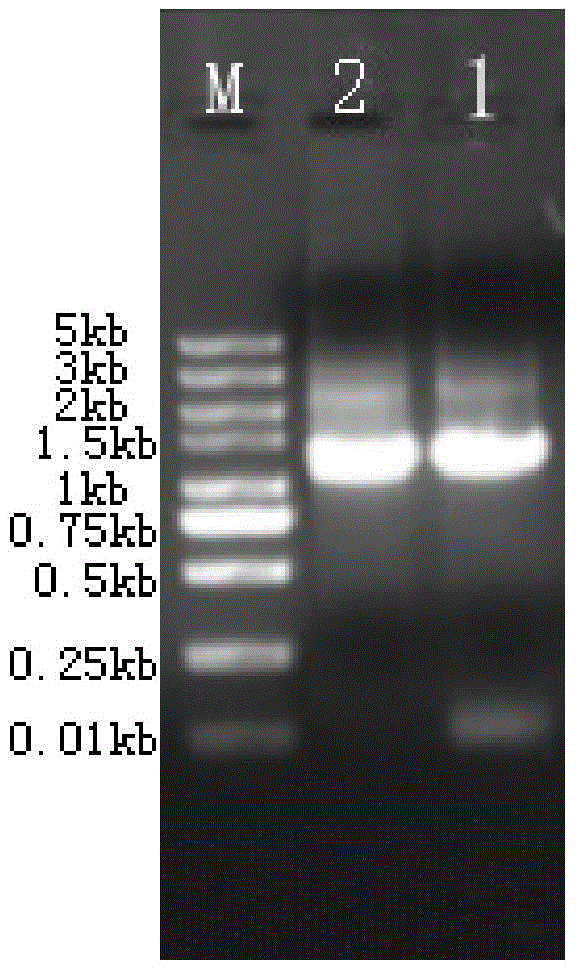
[0051] The pdh gene of Geobacillus sp. Y412MC61 was introduced into E.coli. To amplify the pdh gene (SEQ ID No.2) encoding the PDH enzyme (SEQ ID No.1), use primers F1 (SEQ ID No.3) and R1 (SEQ ID No.4) as forward and reverse, respectively primers. Both primers contained sites compatible with the PCR-amplified deoC gene fragment obtained by site-directed recombination cloning using Gateway Technology. The gel electrophoresis patterns of the PCR amplification products are as follows: figure 1 shown. Using a random mutagenesis kit, by changing the MnSO 4 concentration to carry out multiple reactions, thereby introducing 1 to 3 point mutations into the pdh gene (SEQ ID No.2) of Geobacillus sp.Y412MC61, so that 1-3 amino acid residues in the amino acid sequence of the PDH enzyme are replaced.
[0052] Sequence of forward primer F1 (SEQ ID No.3):
[0053] 5'-GAGCATATGAATGTCATGCTATCGCC-3'
[0054] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com