Apramycin liposome and preparation method thereof
A technology of apramycin liposome and apramycin, which is applied in the field of apramycin liposome and its preparation, can solve the problems of liposome particle size, small action force, and easy aggregation, and achieve small particle size , avoid oxidation, overcome the effect of easy reunion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
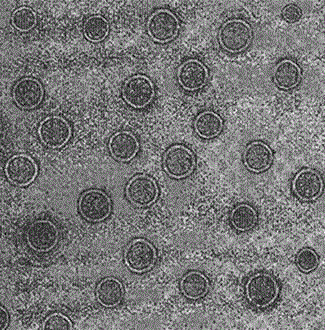
Image
Examples
Embodiment 1
[0031] Weighed 3.75 g of phosphatidylcholine, purchased from Shanghai Boao Bioengineering Company, analytically pure, lot number BA20121206; cholesterol 1.25 g, provided by Shanghai Bioengineering Company, analytically pure, lot number BA20121125; apramycin 0.25 g, Zhengzhou Furuikang Chemical Products Co., Ltd., batch number 120115; dissolve the weighed phosphatidylcholine, cholesterol and apramycin in 13mL of absolute ethanol, mix well to obtain a mixed solution, and place the resulting mixed solution in CO 2 Fluid reactor, capped.
[0032] Poloxamer 188 was dissolved in PBS phosphate buffer solution with pH 7.2 to prepare 100 mL of a stabilizer solution with a mass concentration of Poloxamer 188 of 5%, and the prepared stabilizer solution was placed in CO 2 Fluid collector, capped.
[0033] Open CO 2 Cylinder, CO 2 After the gas is cooled into a liquid by a refrigerator, it is pressurized by a high-pressure metering pump and enters the CO 2 Fluid Reactor, CO 2The te...
Embodiment 2
[0045] Weigh 6 g of phosphatidylcholine, purchased from Shanghai Boao Bioengineering Company, analytically pure, batch number BA20121206; 0.2 g of apramycin, Zhengzhou Furuikang Chemical Products Co., Ltd., batch number 120115; Alkali and apramycin were dissolved in 15mL ethanol (aqueous solution with a mass fraction of 20%), mixed evenly to obtain a mixed solution, and the resulting mixed solution was placed in CO 2 Fluid reactor, capped.
[0046] Poloxamer 188 and Tween 80 were dissolved in PBS phosphate buffer solution of pH 7.2 to prepare 100 mL of a stabilizer solution. The mass concentrations of Poloxamer 188 and Tween 80 in the stabilizer solution were both 5%. The prepared stabilizer solution was placed in CO 2 Fluid collector, capped.
[0047] Open CO 2 Cylinder, CO 2 After the gas is cooled into a liquid by a refrigerator, it is pressurized by a high-pressure metering pump and enters the CO 2 Fluid Reactor, CO 2 The temperature of the fluid reactor is controll...
Embodiment 3
[0050] Weigh 6 g of phosphatidylcholine, purchased from Shanghai Boao Bioengineering Company, analytically pure, batch number BA20121206; 0.6 g of apramycin, Zhengzhou Furuikang Chemical Products Co., Ltd., batch number 120115; Alkali and apramycin were dissolved in 20mL ethanol (90% aqueous solution), mixed evenly to obtain a mixed solution, and the resulting mixed solution was placed in CO 2 Fluid reactor, capped.
[0051] Dissolve poloxamer 188 and alkyl diphenyl ether sulfonate (DAS) in PBS phosphate buffer at pH 7.2 to prepare 100 mL of stabilizer solution. In the stabilizer solution, poloxamer 188, alkyl diphenyl ether sulfonate The mass concentration of phenyl ether sulfonate (DAS) was 5%, and the prepared stabilizer solution was placed in CO 2 Fluid collector, capped.
[0052] Open CO 2 Cylinder, CO 2 After the gas is cooled into a liquid by a refrigerator, it is pressurized by a high-pressure metering pump and enters the CO 2 Fluid Reactor, CO 2 The temperature...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com