Selaginella pulvinata extract as well as preparation method and applications thereof
A technology of Selaginella cuspidatum and its extract, which is applied in the field of Selaginella cuspidatum extract and its preparation, can solve the problem that there is no research on PDE4 inhibitors, and achieve good inhibitory activity and selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Preparation of Selaginella Cushion Extract
[0045] Take 1 kg of the whole herb of Selaginella cushion-like, and grind it into coarse powder. Add 8 times the volume of 95% ethanol, soak for 7 days, filter under reduced pressure, recover ethanol under reduced pressure, and concentrate the remaining liquid to a thick paste with a relative density of 1.25. The step of soaking and extracting was repeated more than two times to finally obtain 50 g of the ethanol extract of Selaginella cushion. The ethanol extract of Selaginella cuspidatum was dissolved in 1L of water, and extracted three times with equal amounts of petroleum ether and ethyl acetate respectively. The ethyl acetate extracts were combined and concentrated under reduced pressure to obtain 39 g of ethyl acetate extract.
Embodiment 2
[0047] Separation of monomeric compounds
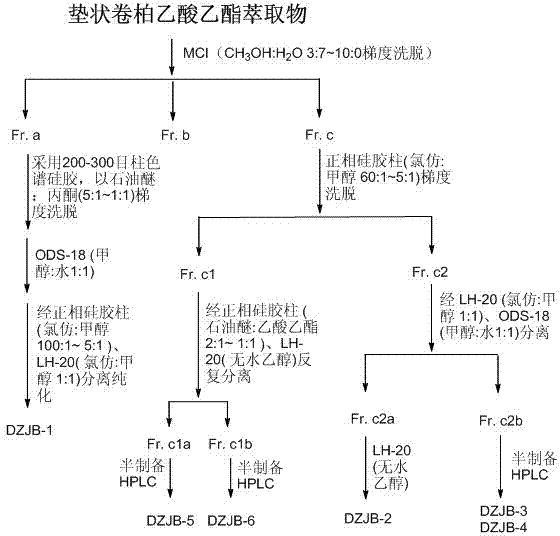
[0048] According to Example 1, 39 g of ethyl acetate extract was obtained, and the ethyl acetate extract was initially segmented by MCI column, and eluted with methanol:water=3:7~10:0; then 200-300 mesh column chromatography silica gel was used to Petroleum ether: ethyl acetate or petroleum ether: acetone or chloroform: gradient elution in solvent systems such as methanol, and then repeatedly use gel column LH-20, ODS column purification, and finally through semi-preparative high performance liquid chromatography (acetonitrile: water) ) or (methanol: water) to further purify, and finally obtain 6 monomeric compounds, the specific separation process refers to figure 1 .
Embodiment 3
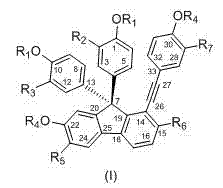
[0050]DZJB-1: Preparation of 4,4'-(7-hydroxy-2-hydroxymethyl-1-(4-hydroxyphenyl)ethynyl-9H-fluorene-9,9-disubstituted)biphenol
[0051] According to the method of Example 2, finally by gel (LH-20 chloroform:methanol=1:1) purification, recrystallization, obtain title compound, yellow bulk crystal, its structure is as follows:
[0052]
[0053] UV(MeOH)λ max (log ε)204(4.84)nm, 300(4.47)nm.
[0054] IR(KBr)ν max 3312, 2207, 1607, 1510, 1449, 1357, 1241, 1174 and 833cm -1 .
[0055] Negative ESIMSm / z511.2[M-H] - ,546.9[M+Cl] - ,556.7[M+HCOO] - ;HRESIMS m / z511.1543[M+H] + (calcd for C 34 h 24 o 5 , 511.1551).
[0056] 4,4'-(7-Hydroxy-2-hydroxymethyl-1-(4-hydroxyphenyl)ethynyl-9H-fluorene-9,9-disubstituted)biphenol spectral data (400MHz, Methanol-d4)
[0057]
[0058]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com