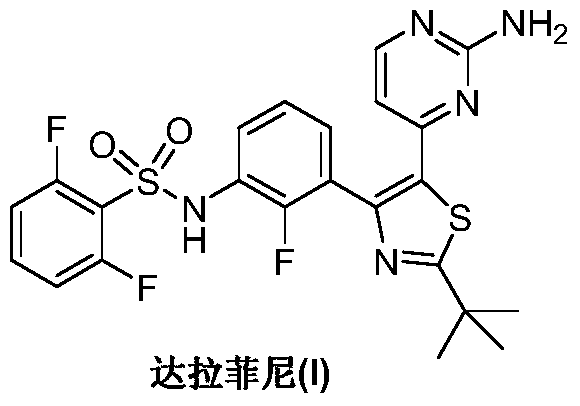
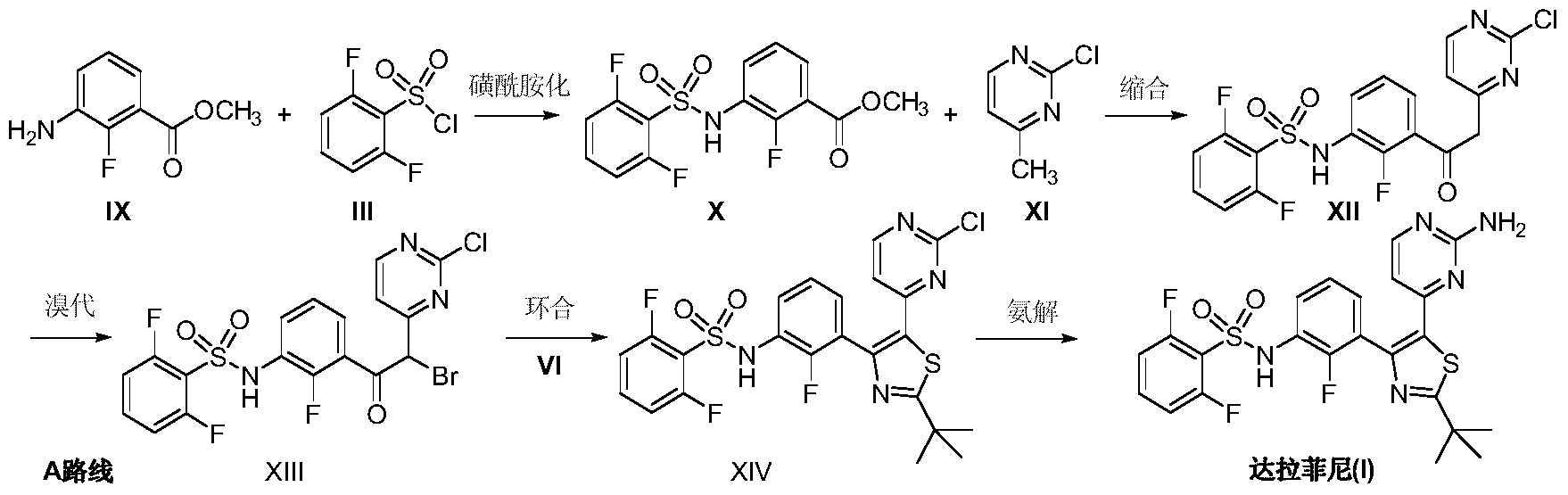
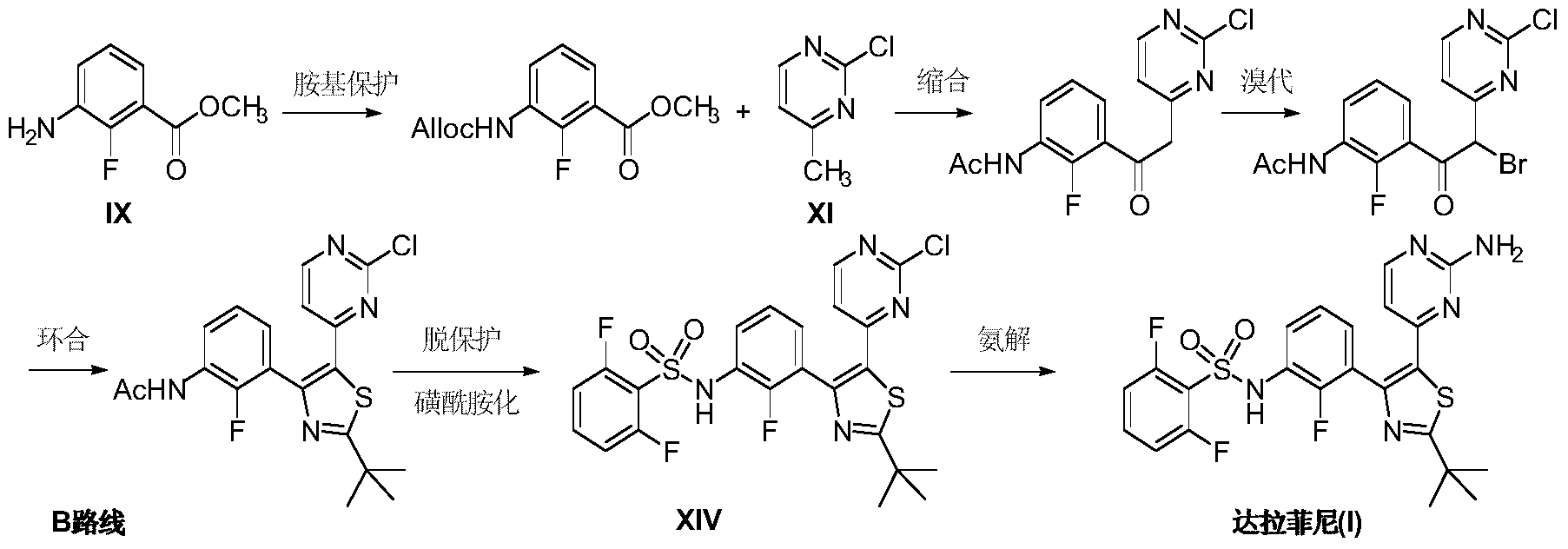
Preparation method of dabrafenib
A fluorophenyl and reaction technology, applied in the field of preparation of dabrafenib, can solve the problems of harsh reaction conditions, many reaction steps, difficult post-processing, etc., and achieve the effects of controllable production, improved product quality, and improved atom economy.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Add 3-(3-amino-2-fluorophenyl)-3-oxa-propionic acid ethyl ester (II) (11.3g, 50mmol), pyridine 10mL, catalytic amount of 4-dimethylaminopyridine into the three-necked flask (DMAP) and dichloromethane 200mL, after stirring and dissolving, add dropwise 2,6-difluorobenzenesulfonyl chloride (III) (11.7g, 55mmol) in dichloromethane 50mL solution at 0°C, after dropping, react at room temperature for 16 After 1 hour, TLC detected that the reaction was over. Add water to wash, and the obtained organic phase is washed with brine, dried over anhydrous sodium sulfate, and the solvent is recovered by distillation under reduced pressure. The obtained crude product is washed with petroleum ether to obtain an off-white solid 3-[3-(2,6-difluorobenzenesulfonamide 12.4 g of ethyl)-2-fluorophenyl]-3-oxa-propionic acid (IV), and the yield was 60.5%.
Embodiment 2
[0032] To 3-[3-(2,6-difluorobenzenesulfonamido)-2-fluorophenyl]-3-oxa-propionic acid ethyl ester (IV) (10g, 25mmol) and dichloromethane 150mL was added N-bromosuccinimide (NBS) (8.9 g, 50 mmol), the resulting red solution was stirred at room temperature for 30 minutes, then concentrated under reduced pressure, and the obtained residue was dissolved in 100 mL of dioxane. Magnesium carbonate (2.1, 25mmol) and 2,2-dimethylthiopropionamide (VI) (3.5g, 30mmol) were added to the solution, and after stirring at room temperature for 4 hours, the reaction was quenched with water and 1N hydrochloric acid, and The reaction was stirred for 0.5 hours. Filtration, the resulting solid was recrystallized from ethyl acetate and n-hexane (1:1) to give white solid N-[3-[5-ethyl carboxylate-2-(tert-butyl)-4-thiazolyl]-2-fluoro 7.25 g of phenyl]-2,6-difluorobenzenesulfonamide (VII), the yield is 58.2%.
Embodiment 3
[0034] Add N-[3-[5-ethyl carboxylate-2-(tert-butyl)-4-thiazolyl]-2-fluorophenyl]-2,6-difluorobenzenesulfonamide (VII) into the reaction flask (6.0g, 12mmol), sodium methoxide (0.81g, 15mmol) and anhydrous ethyl acetate 50mL. Stir at room temperature for 1 hour, heat up to 80°C, reflux for 5 hours, cool down, and stand at room temperature for 12 hours. Add 100 mL of water, add 50 mL of concentrated hydrochloric acid while stirring, raise the temperature to reflux, and react for 4 hours. TLC detects that the reaction is complete. The pH was adjusted to 9-10 with 2M sodium hydroxide. Extracted 3 times with chloroform, combined the organic phases, dried over anhydrous sodium sulfate, and recovered the solvent by distillation under reduced pressure to obtain the product N-[3-(5-acetyl-2-tert-butyl-4-thiazolyl)-2-fluoro 5.4 g of phenyl]-2,6-difluorobenzenesulfonamide (VIII). It can be directly used in the following reaction without purification.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com