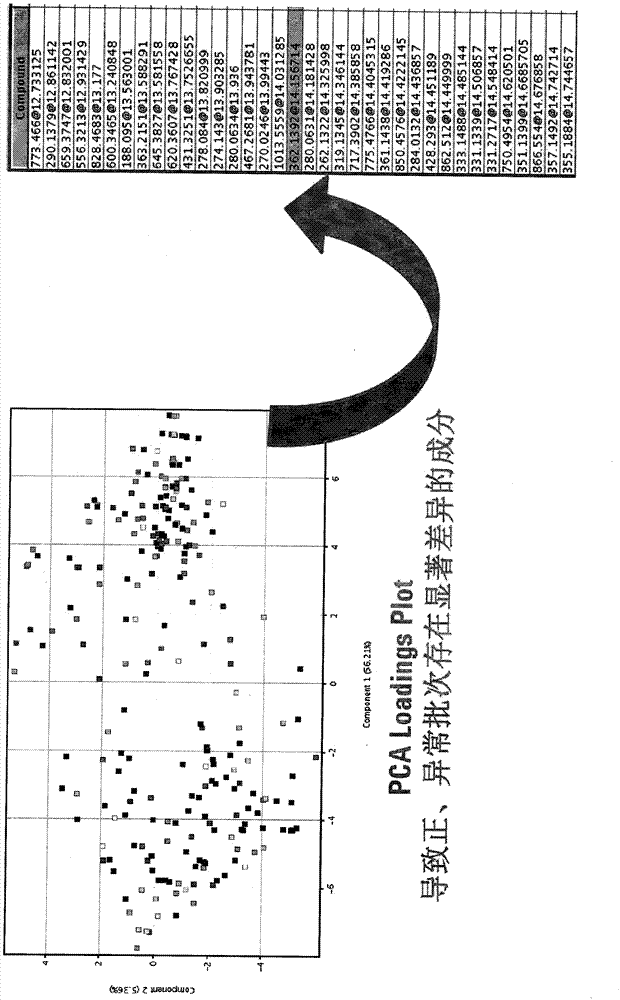
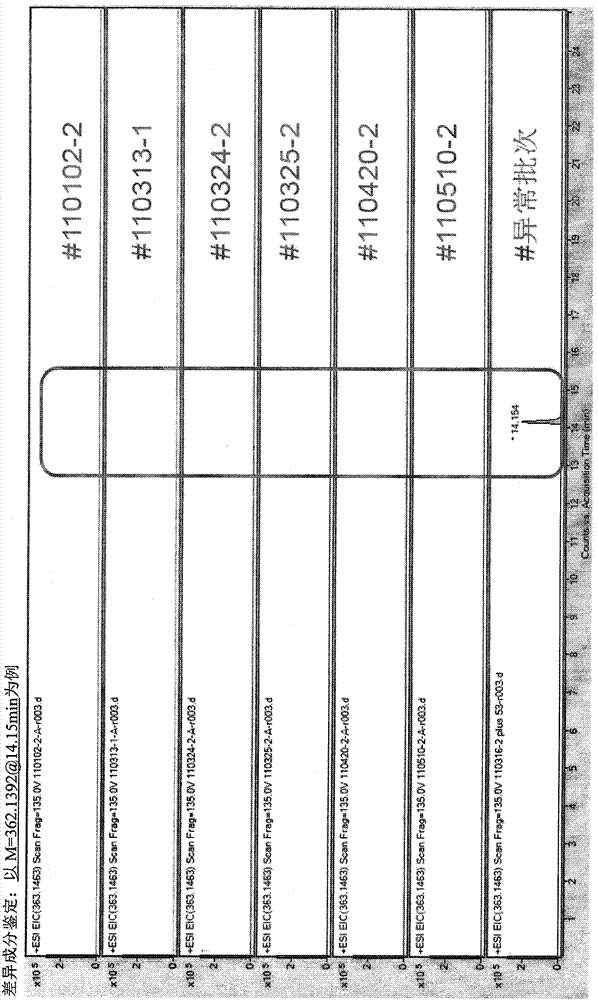
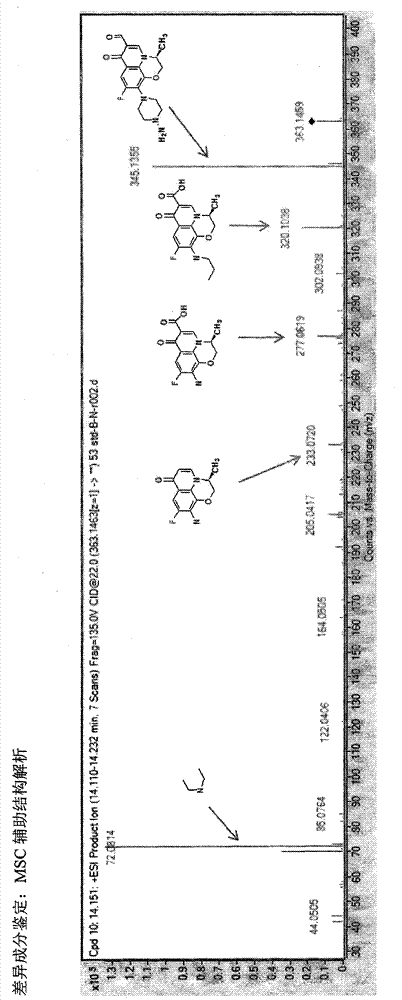
Novel quality control method of Shuxuetong injection
A technology of injection and Shuxuetong, which is applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve the problems of high accuracy, imperfect chemical measurement processing methods, uncertain fingerprints, etc., to achieve high accuracy and avoid defects Response to the effect of batch products entering the market and simple methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Instruments: Agilent 1290 liquid chromatograph (HPLC), Agilent 6530 high-resolution quadrupole time-of-flight tandem mass spectrometer (Q-TOF MS)
[0037] High performance liquid chromatography separation conditions: choose Jielun SB-Aq chromatographic column; mobile phase: A is 0.1% formic acid aqueous solution, mobile phase B is acetonitrile, gradient eluent, calculated by volume ratio, 0min-8min, 1% mobile phase Phase B, at 12min, 11% mobile phase B, at 20min, 30% mobile phase B, at 25min, 99% mobile phase B, at 30min, 99% mobile phase B; flow rate 0.3ml / min;
[0038] Mass spectrometry detection conditions: positive ion mass spectrometry mode, using electrospray ionization source (ESI), atomization gas and drying gas are nitrogen, where atomization pressure is 55psi, drying gas flow rate is 6.0L / min, drying gas temperature is 350°C, The sheath gas is nitrogen, the flow rate is 11L / min, the temperature is 350°C, the capillary high voltage is 3500V, the fragmentation v...
Embodiment 2
[0042] Instruments: Agilent 1290 Liquid Chromatography (HPLC), Agilent Diode Array Detector (G4212B-DAD), Evaporative Light Scattering Detector (380-ELSD)
[0043] High performance liquid chromatography separation conditions: choose HILIC chromatographic column; mobile phase: A is 20mm ammonium acetate aqueous solution, mobile phase B is acetonitrile, gradient eluent, calculated by volume ratio, 0min-5min, 95% mobile phase B, At 15 minutes, 50% mobile phase B, at 25 minutes, 50% mobile phase B; flow rate 0.3ml / min;
[0044] UV detection wavelength: 254nm.
[0045] ELSD conditions: spray chamber temperature 40°C; evaporation chamber temperature 90°C; gas flow rate 1.6SLM; LED intensity 100%; filter 3.0%; gain value 1.0.
[0046] Results: Using HILIC chromatographic column and selected chromatographic conditions, combined with ultraviolet detector and evaporative light scattering detector, the detection of all components of Shuxuetong injection with and without ultraviolet abso...
Embodiment 3
[0048] Instruments: Waters ACQUITY UPLC liquid chromatograph, Waters Xevo G2-S high-resolution quadrupole time-of-flight tandem mass spectrometer (Q-TOF MS)
[0049] High performance liquid chromatography separation conditions: choose ACQUITY UPLC BEH C 18 Chromatographic column; mobile phase: A is acetonitrile: water=90:10+0.1% formic acid+10mM ammonium acetate, mobile phase B is water+0.1% formic acid+10mM ammonium acetate, gradient eluent, calculated by volume ratio, 0min- 0.3min, 99% mobile phase B, 15min, 55% mobile phase B, 16min, 1% mobile phase B, 17min, 1% mobile phase B, 17.5min, 99% mobile phase B , 20min, 99% mobile phase B; flow rate 0.3ml / min;
[0050] Mass spectrometry detection conditions: positive ion mass spectrometry mode, using electrospray ion source (ESI), atomization gas and drying gas are nitrogen, capillary voltage is 3500V, cone voltage is 45V, ion source temperature is 150°C, desolvation gas temperature is 450°C, The desolvation gas flow rate is 90...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com