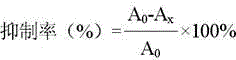Drug for treating purine metabolic disorder disease
A technology of xanthine oxidase and derivatives, which is applied in the application field of drugs for treating purine metabolic disorders, flavonoids and their modified derivatives, can solve the problems of long treatment period, achieve rapid curative effect, abundant drug sources, inhibit Effect of Xanthine Oxidase Activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment 1: The situation that each solvent part of Smilax smilax inhibits XOD activity
[0043] Detection principle: XOD catalyzes xanthine to generate UA, and UA reacts with NBT (nitro blue tetrazolium chloride) to generate purple formazan. When the activity of XOD is inhibited, the amount of UA generated decreases, and purple formazan The generation of cum is reduced accordingly, and the size of the inhibitory activity is detected by measuring the absorbance of the generated purple formazan at 560nm.
[0044] Sample preparation: take 100 g of Smilax smilax, coarsely crush it, pass through a 40-mesh sieve, and use 15 times the amount, 10 times the amount, and 5 times the amount of 50% ethanol to extract 3 times, combine the percolation fluid, and recover until it has no alcohol smell. Dilute to 250ml with distilled water, which is the Smilax tuckahoe sample solution. Measure 25ml of Smilax smilax sample and place it in a separatory funnel, extract with petroleum et...
Embodiment 2
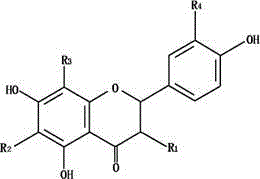
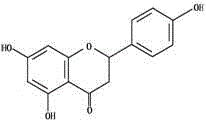
[0051] Example 2: Extraction and Purification of Astilbin in Smilax Smilax
[0052] Take 100g of Smilax smilax medicinal material, coarsely crush it, pass through a 40-mesh sieve, extract 3 times by percolation with 15 times, 10 times and 5 times of 50% ethanol, combine the percolation liquid, concentrate (30°C, d=1.15-1.20 ), add 95% ethanol twice the volume of the concentrated solution, stir evenly to produce a flocculent precipitate, then put it in a refrigerator at 4°C for 12h, and then centrifuge at 4000r / min for 30min. The supernatant was taken, and the ethanol was removed by rotary evaporation. Then wash twice with an appropriate amount of petroleum ether, dry, add 500ml of 50% ethanol to dissolve, and use polyamide resin adsorption method to purify. Water, 10% ethanol, and 50% ethanol were used as eluents respectively, and the 50% ethanol eluent was collected, concentrated and evaporated to dryness to obtain a light yellow substance. Through NMR, SM, IR and UV spectr...
Embodiment 3
[0053] Example 3: Influence on the inhibition of serum UA and XOD activities in rats with hyperuricemia.
[0054] All the rats in the test were SD rats, half of them were male and half were male. A total of 80, weighing 200-240g. 80 rats were taken and randomly divided into 4 groups including blank group, model group, drug group and colchicine group, 20 rats in each group. Except the blank group, the other groups were established with adenine 100mg / kg orally for one week. On the 5th day, astilbin 40 mg / kg and colchicine 40 mg / kg were given by intragastric administration to the drug group and colchicine group, respectively, for 5 consecutive days. The blank group and the model group were given equal volumes of distilled water, and each group was given 0.25ml / 25g body weight. On the 10th day of the experiment, 1 hour after the administration, the animals in each group were removed from the eyeballs to get blood, and the activities of UA and XOD in the serum of the rats were m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com