Method for preparing nano calcium peroxide by chemical modification
A calcium peroxide, chemical modification technology, applied in chemical instruments and methods, nanotechnology for materials and surface science, inorganic chemistry, etc. The effect of increasing adsorption sites, large specific surface area, and more reaction sites
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
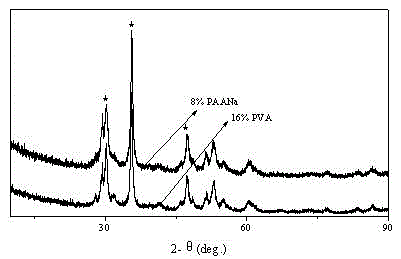
[0027] (1) Accurately weigh 6g of calcium hydroxide, dissolve it in 120mL of deionized water, and stir to form a calcium hydroxide suspension. Weigh 0.96 g (that is, 16% of the mass fraction of calcium hydroxide) of polyvinyl alcohol into the suspension, and ultrasonically and mechanically stir for 50 minutes at room temperature to fully mix the above substances to form an inorganic-polymer reaction substrate.
[0028] (2) 63.46mL30% hydrogen peroxide was added to a 100mL headspace bottle, sealed with a capper, inserted into a burette with a controllable flow rate and slowly dripped into the bottom liquid. The reaction was carried out under low temperature conditions with constant uniform stirring. After the dripping is completed, the reaction is continued for 15 minutes to fully react the calcium salt and hydrogen peroxide to form stable nano-scale calcium peroxide particles.
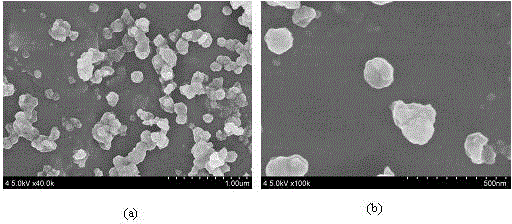
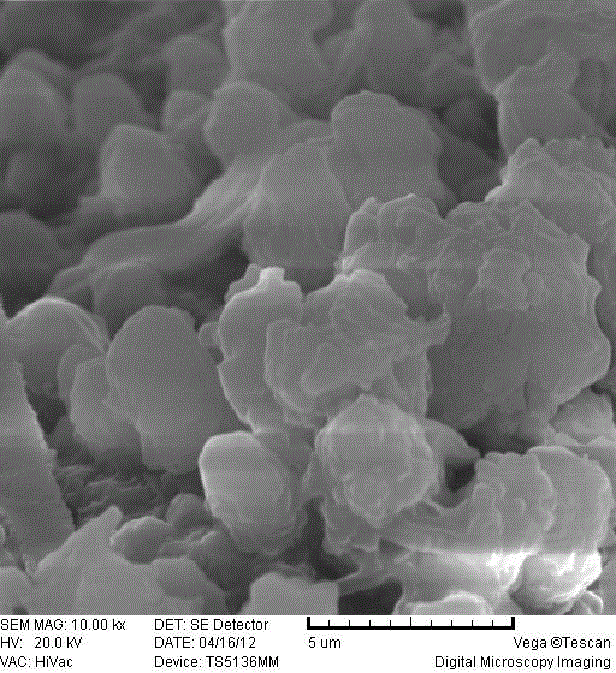
[0029] (3) The formed precipitate is centrifuged, and the product is washed with deionized water and iso...
Embodiment 2
[0032] (1) Accurately weigh 6g of calcium hydroxide, dissolve it in 120mL of deionized water, and stir to form a calcium hydroxide suspension. Weigh 0.48g (ie 8% of the mass fraction of calcium hydroxide) polyvinyl alcohol and add it to the suspension, ultrasonically and mechanically stir for 30min at room temperature to fully mix the above-mentioned substances to form an inorganic-polymer reaction substrate.
[0033] (2) 63.46mL30% hydrogen peroxide was added to a 100mL headspace bottle, sealed with a capper, inserted into a burette with a controllable flow rate and slowly dripped into the bottom liquid. The reaction was carried out under low temperature conditions with constant uniform stirring. After the dripping is completed, the reaction is continued for 20 minutes to make the calcium salt and hydrogen peroxide fully react to form stable nano-scale calcium peroxide particles.
[0034] (3) The formed precipitate is centrifuged, and the product is washed with deionized water and...
Embodiment 3
[0037] (1) Accurately weigh 6g of calcium hydroxide, dissolve it in 120mL of deionized water, and stir to form a calcium hydroxide suspension. Weigh 0.24g (ie 4% of the mass fraction of calcium hydroxide) of sodium polyacrylate into the suspension, ultrasonically and mechanically stir for 50 minutes at room temperature to fully mix the above substances to form an inorganic-polymer reaction substrate.
[0038] (2) 63.46mL30% hydrogen peroxide was added to a 100mL headspace bottle, sealed with a capper, inserted into a burette with a controllable flow rate and slowly dripped into the bottom liquid. The reaction was carried out under low temperature conditions with constant uniform stirring. After the dripping is completed, the reaction is continued for 15 minutes to fully react the calcium salt and hydrogen peroxide to form stable nano-scale calcium peroxide particles.
[0039] (3) The formed precipitate is centrifuged, and the product is washed with deionized water and isopropanol, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com