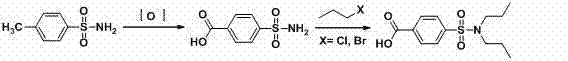
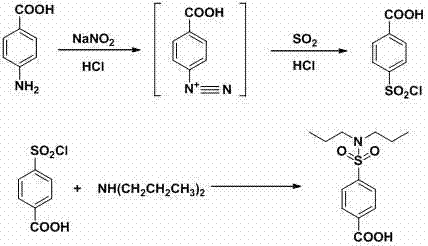
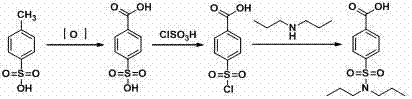
Water-phase synthetic method of probenecid
A probenecid and dipropylamine technology, applied in the field of organic chemical synthesis, can solve the problems of difficult disposal of waste water, restricted development, low yield and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] (1) Diazotization reaction
[0041] Take 68.6g of p-aminobenzoic acid (0.5mol), 250g of water and 127.4ml of hydrochloric acid (31%, 1.25mol) into a 2000ml three-neck flask, stir in an ice-water bath, cool down to 0-5°C, and add sodium nitrite dropwise Solution (34.5g sodium nitrite, 0.5mol, dissolved in 190g water), control the temperature at 10-20°C, control the dropping time for 4 hours, continue to react at this temperature for 1 hour after the dropping, and obtain diazotization The reaction solution.
[0042] (2) Sulfonyl chloride reaction
[0043] Add 250g of water and 765ml of hydrochloric acid (31%, 7.5mol) into a 5000ml three-necked bottle, stir in an ice-water bath, cool down to -5°C, and start feeding liquid sulfur dioxide, controlling the temperature at -3--1°C, when the When 64g of sulfur dioxide (1mol), the absorption of sulfur dioxide is completed, and the sulfonyl chloride reagent is obtained.
[0044] Add the diazotization reaction solution to the su...
Embodiment 2
[0048] (1) Diazotization reaction
[0049] Take 68.6g of p-aminobenzoic acid (0.5mol), 250g of water and 152.9ml of hydrochloric acid (31%, 1.5mol) into a 2000ml three-neck flask, stir in an ice-water bath, cool down to 0-5°C, and add sodium nitrite dropwise Solution (36.0g sodium nitrite, 0.52mol, dissolved in 190g water), control the temperature at 0-10°C, control the dropping time for 3 hours, continue to react at this temperature for 1 hour after the dropping, and obtain diazotization The reaction solution.
[0050] (2) Sulfonyl chloride reaction
[0051] Add 250g of water and 887ml of hydrochloric acid (31%, 8.7mol) into a 5000ml three-necked bottle, stir in an ice-water bath, cool down to -5°C, and start feeding liquid sulfur dioxide, control the temperature at 0-5°C, when 112g of sulfur dioxide (1.75mol), the absorption of sulfur dioxide is complete, and the sulfonyl chloride reagent is obtained.
[0052] Add the diazotization reaction solution to the sulfonyl chloride...
Embodiment 3
[0056] (1) Diazotization reaction
[0057] Take 68.6g of p-aminobenzoic acid (0.5mol), 250g of water and 203.9ml of hydrochloric acid (31%, 2mol) into a 2000ml three-necked flask, stir in an ice-water bath, cool down to -10--5°C, and add nitrous acid dropwise Sodium solution (38.0g sodium nitrite, 0.55mol, dissolved in 190g water), control the temperature at 0-10°C, control the dropping time for 5 hours, continue to react at this temperature for 1 hour after the dropping, and obtain diazo chemical reaction solution.
[0058] (2) Sulfonyl chloride reaction
[0059] Add 250g of water and 968ml of hydrochloric acid (31%, 9.5mol) into a 5000ml three-necked bottle, stir in an ice-water bath, cool down to -5°C, start to feed liquid sulfur dioxide, control the temperature at 5-10°C, when 160g of sulfur dioxide (2.5mol), the absorption of sulfur dioxide is completed, and the sulfonyl chloride reagent is obtained.
[0060] Add the diazotization reaction solution to the sulfonyl chlo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com