Vildagliptin preparation method
A technology of organic solvent and chloroacetyl group, applied in the field of preparation of pharmaceutical compounds, can solve problems such as the harm of fluoroboric acid, high price, etc., and achieve the effects of improving yield, improving yield, and being beneficial to the industrialization of the reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
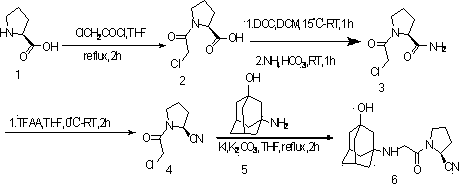
[0038] The present invention provides a new preparation method of vildagliptin, the synthetic route is as follows:
[0039]
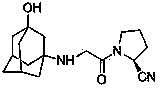
[0040] Wherein 1 is L-proline; 2 is (S)-1-(2-chloroacetyl)pyrrolidine-2-carboxylic acid; 3 is (S)-1-(2-chloroacetyl)pyrrolidine-2 -formamide; 4 is (S)-1-(2-chloroacetyl)pyrrolidine-2-carbonitrile; 5 is 3-aminoadamantanol; 6 is 1-[[(3-hydroxy-1-adamant Alkyl)amino]acetyl]-2-cyano-(5)-tetrahydropyrrolidine (vildagliptin).
[0041] The preferred preparation method of the present invention comprises the following steps:
[0042] 1. Preparation of (S)-1-(2-chloroacetyl)pyrrolidine-2-carboxylic acid (2):
[0043] A certain amount of L-proline and THF are formed into a suspension, and a certain amount of chloroacetyl chloride is slowly added dropwise to the suspension. After the dropwise addition, reflux at 35°C for 2h, dilute with water, and continue to stir for 20min. After the reaction was completed, a certain amount of water was added, and the aqueo...
Embodiment 1
[0054] 1. Preparation of (S)-1-(2-chloroacetyl)pyrrolidine-2-carboxylic acid (2):
[0055] 4.8g (41.62mmol) L-proline and 45mL THF formed a suspension, and 4.7mL (62.43mmol) of chloroacetyl chloride was slowly added dropwise to the suspension. After the dropwise addition, reflux at 35°C for 2h, add 25ml of water to dilute, continue to stir for 20min, extract with 25mL of saturated saline and 50mL of ethyl acetate, extract the aqueous layer with 25*3mL of ethyl acetate, and dry over anhydrous magnesium sulfate. Suction filtration, rotary evaporation of the filtrate to obtain an oily liquid. Diethyl ether was recrystallized, cooled and filtered to obtain 7.21 g of a white solid with a yield of 91%.
Embodiment 2
[0057] 2. Preparation of (S)-1-(2-chloroacetyl)pyrrolidine-2-carboxamide (3):
[0058] (S)-1-(2-chloroacetyl)pyrrolidine-2-carboxylic acid 5g (0.026mol) was dissolved in 50mL of CH 2 Cl 2 Stirring in , DCC (5.4g, 0.026mol) was dissolved in 50mL CH 2 Cl 2 middle. CH of DCC 2 Cl 2 The solution was slowly added dropwise to the CH of formic acid 2 Cl 2 In the solution, it is expected to add dropwise for 1 hour, and after the dropwise addition, continue to react for 1 hour. Join NH 4 HCO 3 (20.6g, 0.26mol) continue reaction 1h, TLC detects developing agent (CH 2 Cl 2 : MeOH=20:1), after the reaction is complete, filter with CH 2 Cl 2 The filter residue was washed, the solution was concentrated, a small amount of ethyl acetate was added to recrystallize and cool the liquid, and the crude weight was 3.41 g by suction filtration, with a yield of 69%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com