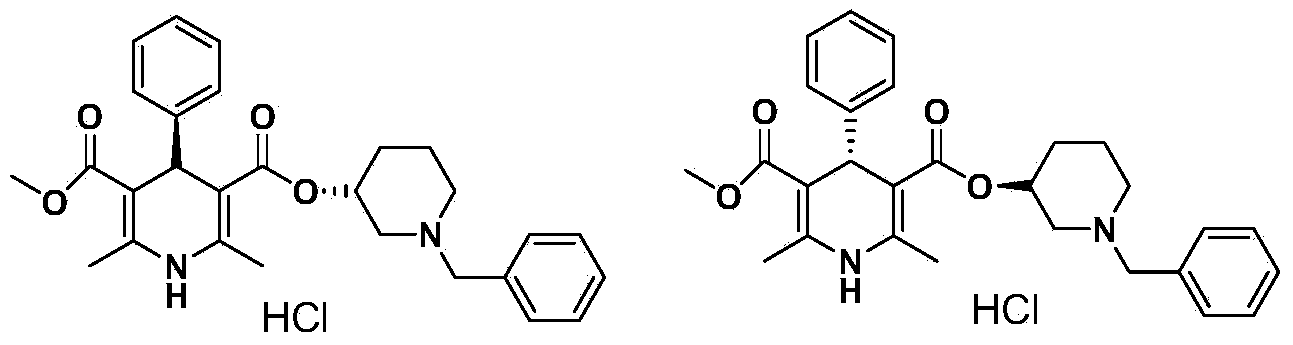
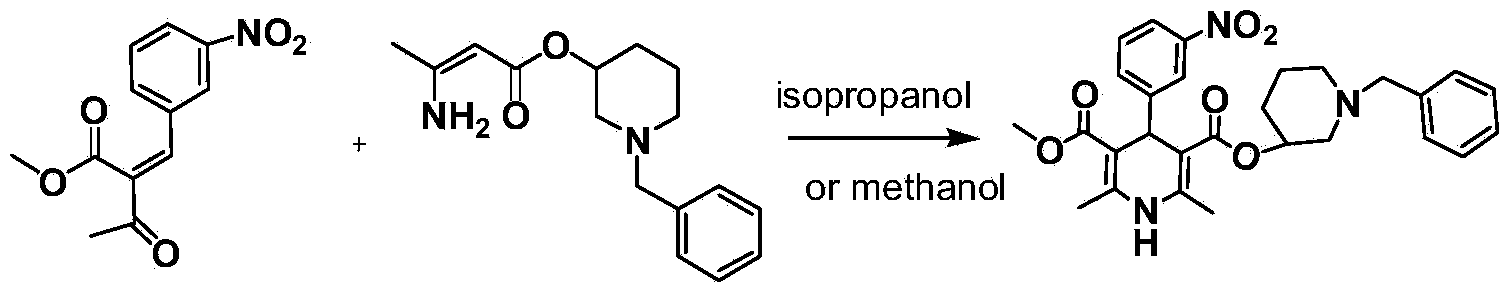Preparation method of benidipine hydrochloride
A technology of benidipine hydrochloride and carboxylic acid, applied in the field of pharmaceutical synthesis, can solve problems such as difficult industrial scale-up production, difficult purification, long reaction time, etc., and achieves a technology that is beneficial to industrial production, has no potential safety hazard and stable reaction yield. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
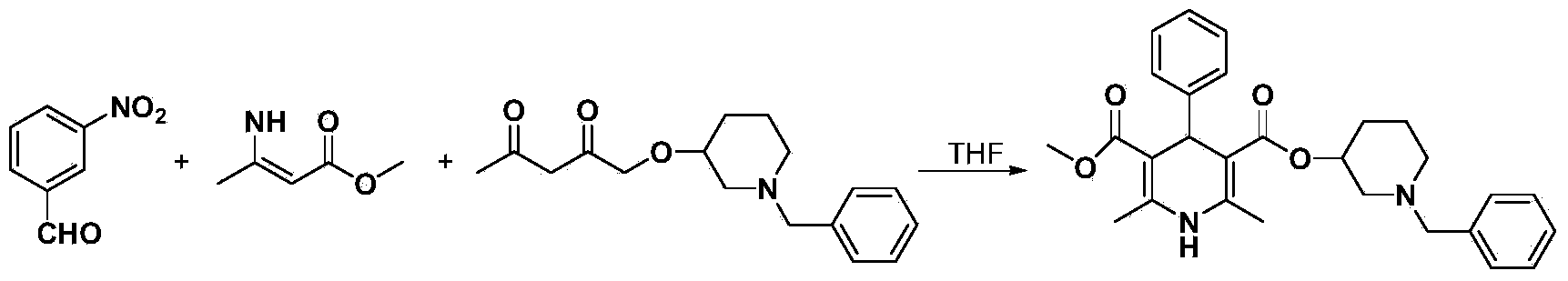
Embodiment 1
[0052] In the present invention, the N-benzyl-3-hydroxypiperidine used in step 2) can be synthesized by the following method: 27.4 grams (0.198mol, 2.0eq) of potassium carbonate are suspended and dissolved in 50ml DMF, and in the solution Add 10.0 g (0.0988 mol, 1.0 eq) of 3-hydroxypiperidine and 18.6 g (0.108 mol, 1.09 eq) of benzyl bromide, stir at 60°C for 3 days, monitor the disappearance of 3-hydroxy piperidine by TLC, and then cool down to room temperature. Add 300 ml of water to the reaction solution and stir for 30 minutes, extract with ethyl acetate, add about 150 ml of water for extraction three times, combine the organic layers, wash with about 50 ml of water, and wash the organic layer with anhydrous sodium sulfate Dry, filter and spin dry to obtain 15.1 g of product, the yield is 79.7%, and the product is light yellow oil. The N-benzyl-3-hydroxypiperidine that obtains is carried out the identification of proton magnetic spectrum: 1 H NMR(500MHz,CDCl3),δ1.48-1.55(...
Embodiment 2
[0054] Add 50 grams (0.15mol, 1.0eq) of 2,6-dimethyl-4-(m-nitrophenyl)-1,4-dihydropyridine-5-carboxylic acid methyl ester-3-carboxylic acid to 500 Add 18.2 grams (0.18mol, 1.2eq) of triethylamine to milliliter of dichloromethane solution, add 28.54 grams (0.165mol, 1.1eq) of diethyl chlorophosphate, react at 20°C for 1 hour, TLC tracking, raw material reaction After completion, a reaction liquid containing mixed acid anhydride is formed. 25.80 g (0.135 mol, 0.9 eq) of N-benzyl-3-hydroxypiperidine was added to the reaction solution, stirred at 20°C for 5 minutes and then refluxed for 4 hours, during which time TLC was followed. After the reaction was completed, the reaction solution was lowered to 40°C and decolorized by adding activated carbon, filtered, and the filtrate was spin-dried to obtain the crude product of benidipine (since dichloromethane was used as the solvent, the spin-drying was not required, and the next step was directly carried out). After the crude benidipi...
Embodiment 3
[0056] 25 grams (0.075mol, 1.0eq) of 2,6-dimethyl-4-(m-nitrophenyl)-1,4-dihydropyridine-5-carboxylic acid methyl ester-3-carboxylic acid was added in 250 Add 15.15 g (0.15 mol, 2.0 eq) of triethylamine to 1 ml of tetrahydrofuran solution, add 18.2 g (0.105 mol, 1.4 eq) of diisopropyl chlorophosphate, react at 30°C for 2 hours, follow TLC, and the reaction of raw materials is complete Finally, a reaction liquid containing mixed acid anhydride is formed. 15.77 g (0.0825 mol, 1.1 eq) of N-benzyl-3-hydroxypiperidine was added to the reaction solution, stirred at 30°C for 15 minutes and then refluxed for 6 hours, during which TLC was followed. After the reaction was completed, the reaction solution was lowered to 50° C., decolorized by adding activated carbon, filtered, and the filtrate was spin-dried to obtain crude benidipine. After the benidipine crude product is dissolved in dichloromethane, it is washed with 6% NaOH solution, water, 2.5mol / L hydrochloric acid and water succes...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com