Compound micelle-based nano-vector, and preparation method and application thereof
A technology of compound micelles and nanocarriers, which can be applied in preparations for in vivo experiments, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the problem of the restriction of the application range of anti-tumor antibiotics, multi-drug resistance Drugs, anti-tumor antibiotics strong cytotoxicity and other issues, to overcome multi-drug resistance, good stability, good biocompatibility and biodegradability effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0037] In a more specific embodiment of the present invention, the preparation method may include the following steps:
[0038] ① Prepare dextran-4-nitro-p-benzochloroformate (DEX-PNC) by precipitation washing and vacuum drying, the purpose is to introduce acid chloride on dextran;
[0039] ② DEX-PNC and cystamine hydrochloride dissolved in water were obtained by dialysis to obtain DEX-SS-NH 2 polymer;
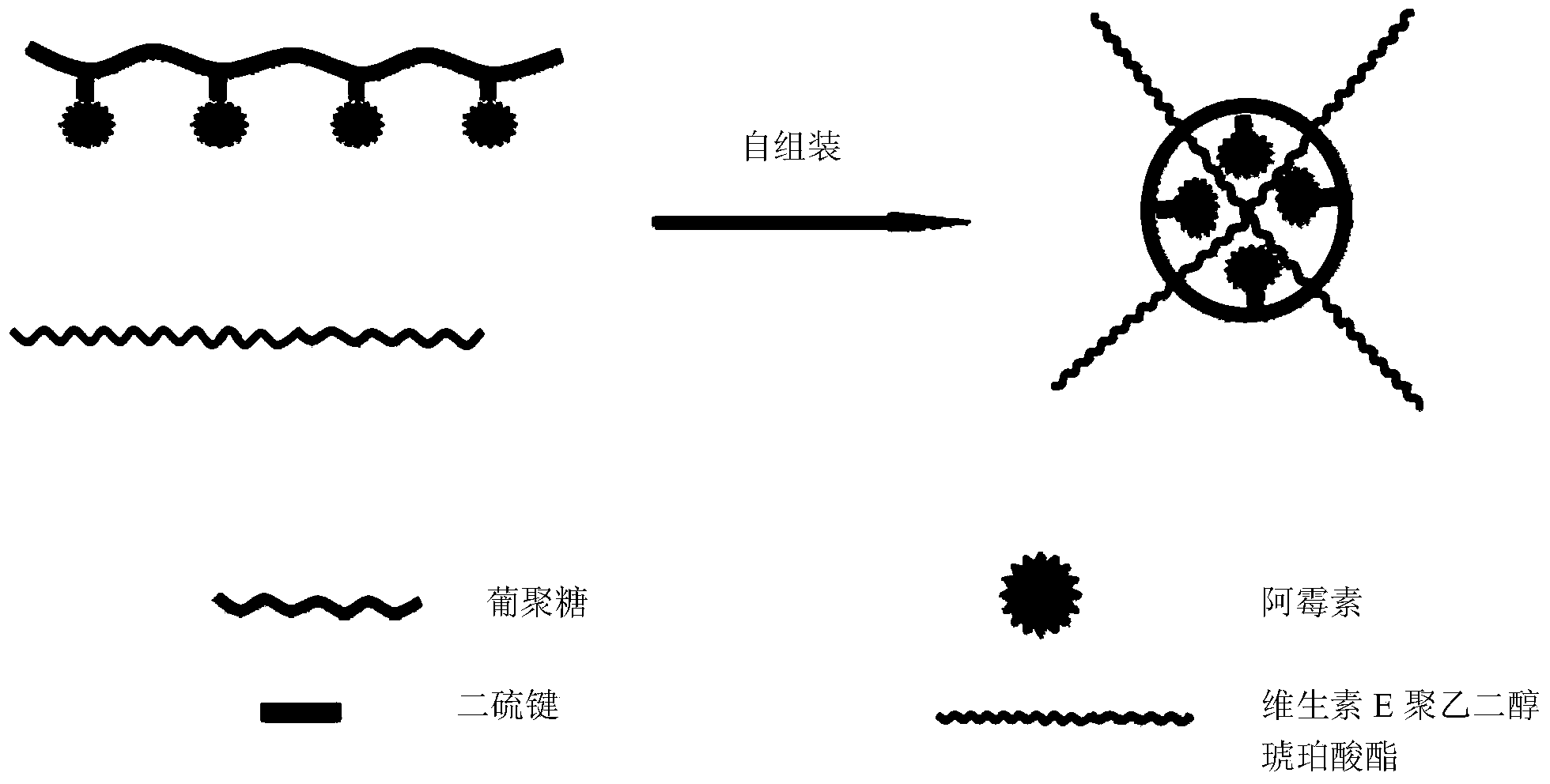
[0040] ③ Active components activated by succinic anhydride and DEX-SS-NH 2 The reduction-responsive dextran-loaded active component is obtained by dialysis, and the reduction-responsive dextran-loaded active component is combined with water-soluble vitamin E to obtain a composite micelle by dialysis.
[0041] Among them, dithiodianhydride can also be used instead of cystamine hydrochloride to obtain reduction-sensitive bonds.
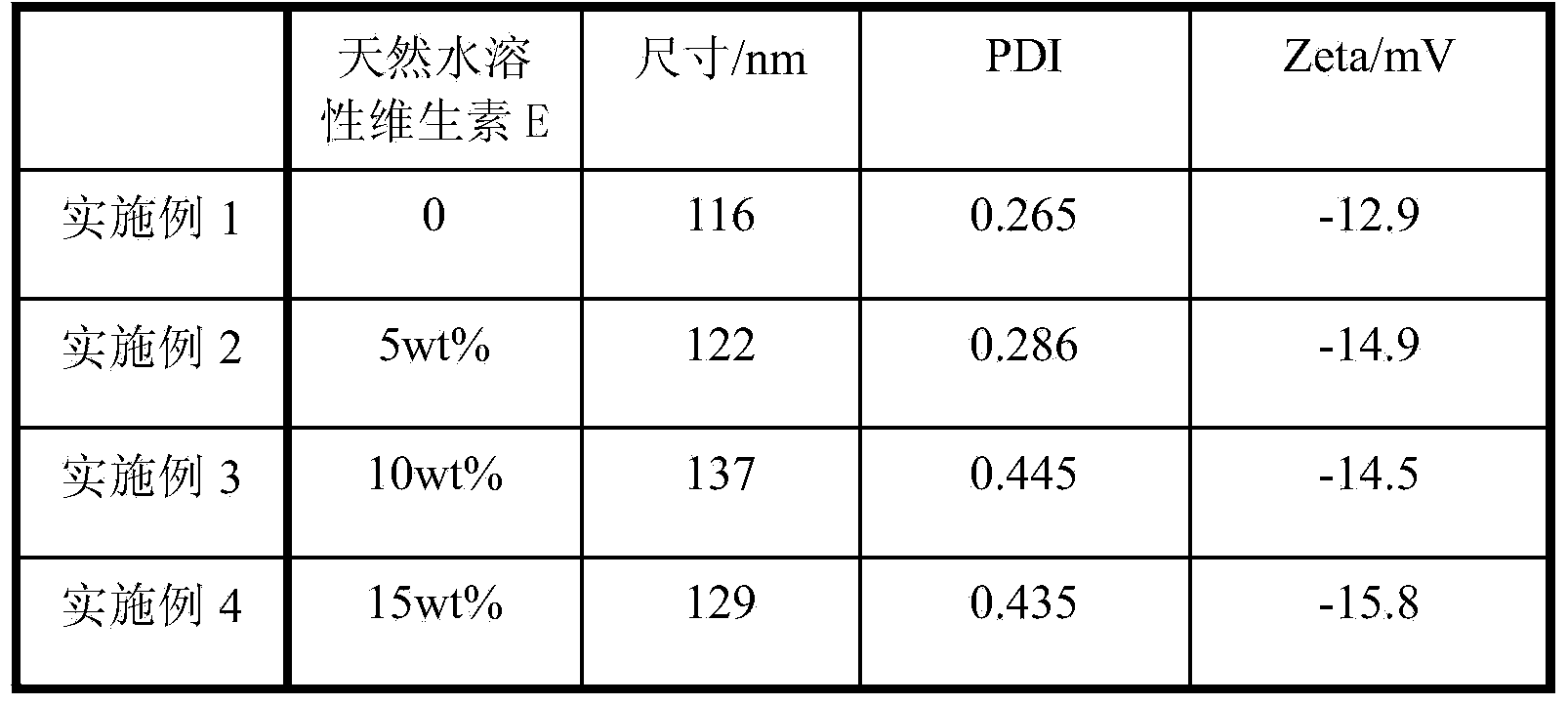
[0042] Moreover, in the present invention, multidrug resistance can be overcome to the greatest extent by adjusting the dosage of water-soluble vita...
Embodiment 1
[0055] Example 1: Preparation of dextran-4-nitro-p-benzochloroformate (DEX-PNC)
[0056]Dissolve 0.3 g of dextran with a molecular weight of 40,000 in 40 mL of dimethyl sulfoxide and pyridine (1:1 by volume) solution, add 0.62 g of 4-dimethylaminopyridine and 0.04 g of 4-nitroparaben chloroformate , react in ice bath for 4h, after the reaction, sink into 10 times the mixture of ethanol and ether (v / v=1 / 1), wash the precipitate, and dry it in vacuum to obtain DEX-PNC polymer. Dissolve 1.6g of cystamine hydrochloride into 30mL of dimethyl sulfoxide solution, react with the DEX-PNC polymer obtained above for 24h at room temperature, and then dialyze to obtain DEX-SS-NH 2 Polymer, the obtained product was reacted with succinic anhydride-activated doxorubicin to obtain DEX-SS-DOX micelles.
Embodiment 2
[0057] Embodiment 2: the preparation of DEX-SS-DOX and TPGS
[0058] The above-mentioned product DEX-SS-DOX and the natural water-soluble vitamin E of 5% by mass ratio obtain composite micelles by the method of dialysis, and its structure can refer to figure 1 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com