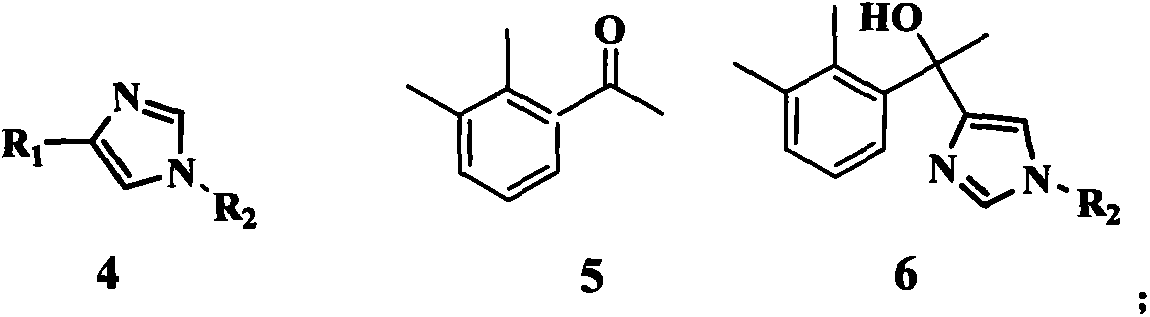
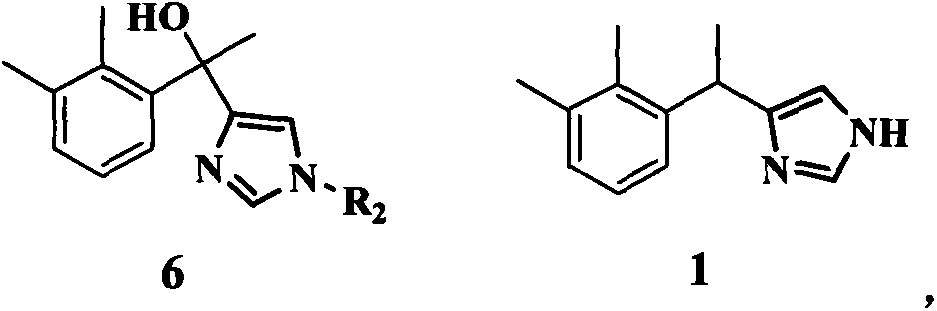
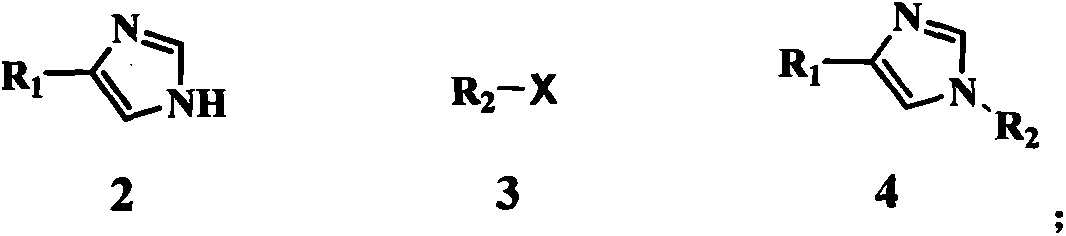Method for preparing medetomidine
A temperature control and compound technology, applied in organic chemistry and other directions, can solve the problems of high cost, long route, and high production equipment requirements, and achieve the effects of easy control, simple process conditions, and efficient preparation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 14
[0056] Embodiment 14-Iodo-N, the preparation of N-dimethyl-1H-imidazole-1-sulfonamide (step 1)
[0057] 4-iodo-imidazole (27g, 0.139mol) and N, N-dimethyl chlorosulfonamide (21g, 0.146mol) were dissolved in 100ml of tetrahydrofuran, and sodium hydroxide (8.4 g, 0.21mol); after feeding, warm to room temperature, react for 12h, add water 50ml, isopropyl acetate 150ml, separate liquid, take oil layer, concentrate to 100ml, add n-hexane 100ml, heat to reflux for 0.5h, cool and analyze crystal, filtered, and dried to obtain 37.9 g of a white solid, namely 4-iodo-N, N-dimethyl-1H-imidazole-1-sulfonamide, with a yield of 90.5%. 1 H-NMR (CDCl 3 , 400MHz): δ7.84(s, 1H), δ7.48(s, 1H), δ2.94(s, 6H).
Embodiment 24
[0058] Example 24-Iodo-N, the preparation of N-dimethyl-1H-imidazole-1-sulfonamide (step 1)
[0059] Dissolve 4-iodo-imidazole (27g, 0.139mol) and N, N-dimethyl chlorosulfonamide (21g, 0.146mol) in 100ml of acetonitrile, and add potassium hydroxide (12g , 0.214mol); after the feeding was completed, it was raised to room temperature, and after 15 hours of reaction, 60ml of water and 200ml of isopropyl acetate were added, the liquid was separated, the oil layer was taken, concentrated to 100ml, 120ml of n-hexane was added, heated to reflux for 0.5h, cooling and crystallization , filtered, and dried to obtain 37.8 g of a white solid, namely 4-iodo-N, N-dimethyl-1H-imidazole-1-sulfonamide, with a yield of 90.3%.
Embodiment 34
[0060] Example 34-Iodo-N, the preparation of N-dimethyl-1H-imidazole-1-sulfonamide (step 1)
[0061] Dissolve 4-iodo-imidazole (27g, 0.139mol) and N,N-dimethylchlorosulfonamide (20g, 0.139mol) in 100ml tetrahydrofuran, and add sodium hydroxide ( 9.3g, 0.25mol); after feeding, raise to 15°C, react for 24h, add 50ml of water, 150ml of isopropyl acetate, separate liquid, take oil layer, concentrate to 100ml, add 100ml of n-hexane, heat and reflux for 0.5h, Cooling and crystallization, filtration, and drying yielded 37.4 g of a white solid, namely 4-iodo-N,N-dimethyl-1H-imidazole-1-sulfonamide, with a yield of 89.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com