Puerarin derivatives and preparation methods thereof
A puerarin derivative and selected technology, applied in its preparation field, can solve the problems of poor water solubility and fat solubility of puerarin, small clinical application range of puerarin, limitation of biological activity and clinical application, etc., and achieve fat solubility Good, good water solubility, high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
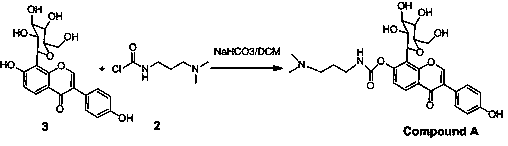
[0028] Synthesis of Example 1 Compound A
[0029] The synthetic route and the structural formula of each compound refer to the attached figure 1 - attached figure 2 .
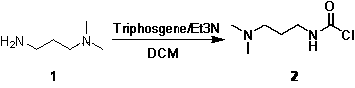
[0030] (1) Synthesis of compound 2
[0031] The synthetic route and the structural formula of each compound refer to the attached figure 1 .
[0032] Dissolve N,N-dimethyl-1,3-diaminopropane (3.06g, 0.03mol) in dichloromethane (150ml), cool down to -10°C, add solid triphosgene (2.97g, 0.01mol) ; Add triethylamine (3.03g, 0.03mol) dropwise under stirring, after the dropwise addition is completed, rise to room temperature and react for 3h;
[0033] After the reaction was completed, add purified water (70ml) to quench the reaction, separate the layers, extract the aqueous layer twice with dichloromethane (70ml*2), combine the organic phases, dry over anhydrous sodium sulfate, and concentrate to obtain an oily substance (4.4g, 90%), the crude product was directly used in the next reaction.
[0034] (2) Sy...
Embodiment 2
[0038] Synthesis of Example 2 Compound B
[0039] The synthetic route and the structural formula of each compound refer to the attached image 3 - attached Figure 7
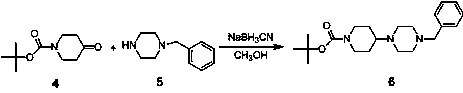
[0040] (1) Synthesis of Compound 6
[0041] The synthetic route and the structural formula of each compound refer to the attached image 3 .
[0042] Dissolve N-tert-butoxycarbonyl-4-piperidone (1.99g, 0.01mol) and N-benzylpiperazine (1.96g, 0.011mol) in methanol (40ml), and quickly add cyanoborohydrogenation under stirring Sodium (1.26g, 0.02mol), react at room temperature, adjust the pH to 7 with acetic acid during the reaction;
[0043] After the reaction was completed, the solvent was evaporated by rotary evaporation, and water (40ml) and dichloromethane (60ml) were added for extraction, the aqueous layer was washed twice with dichloromethane (40ml*2), the combined organic phase was washed with saturated brine, and the organic phase was washed with anhydrous Dry over sodium sulfate and concentrate t...
Embodiment 3
[0059] Example 3 Synthesis of Compound C
[0060] The synthetic route and the structural formula of each compound refer to the attached Figure 8 .
[0061] Put puerarin (4.16g, 0.01mol) and succinic anhydride (1.98g, 0.012mol) into dichloromethane (80ml), add DMAP (100mg), heat to reflux, and react for 24h; 20ml, separated and purified by preparative column, collected fractions, concentrated to 20 mL volume, added sodium bicarbonate to adjust pH to 8, poured the aqueous solution into ethanol (200ml), precipitated white solid, filtered, and vacuum dried to obtain white solid (3.41g, 63.3%).
[0062] At room temperature, the solubility of compound C in water is about 452 mg / ml. Mix a certain amount of compound C with rat anticoagulant plasma, incubate at 37°C, and perform HPLC analysis by solid phase extraction at different time points. The half-life of compound 7 converted into puerarin in blood is determined to be 0.46±0.31h .
PUM
| Property | Measurement | Unit |
|---|---|---|
| Solubility | aaaaa | aaaaa |
| Solubility | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com