Preparation of natural active substance constructed polymer composite medicine and application thereof in inhibiting angiogenesis
A technology of natural active substances and natural macromolecules, applied in the field of biomedical materials, can solve problems such as easy bleeding and restriction, and achieve the effects of easy operation, improved efficiency and improved solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
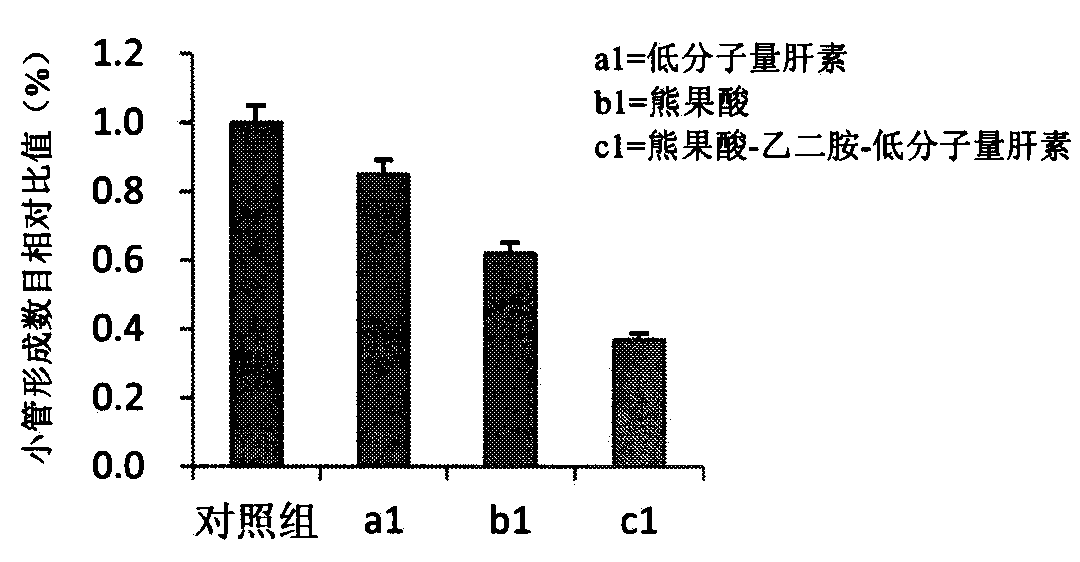
[0063] Embodiment 1: Synthesis of ursolic acid-ethylenediamine-low molecular weight heparin compound medicine
[0064] Weigh an appropriate amount of ursolic acid (UA) and put it in an eggplant-shaped bottle, add 20 mL of tetrahydrofuran, the molar concentration of UA is 0.05 mmol / mL, ice bath, and under the protection of inert gas, add dicyclohexylcarbodiimide in turn (DCC) and hydroxysuccinimide (NHS), the molar ratio of UA, DCC, NHS was 1:1.2:1.2 in turn. The reaction was carried out in an ice bath for 30 min, and then moved to room temperature for 24 h. The precipitated dicyclohexylurea (DCU) was removed by vacuum filtration to obtain a filtrate. The filtrate was precipitated with 3 times the amount of n-hexane for 12 h, filtered and dried in vacuo to obtain succinimidyl UA. Weigh 0.23 g of succinimidyl UA, dissolve it with 15 mL of N,N-dimethylformamide (DMF), and slowly add it dropwise to 1 mL of ethylenediamine for 30 minutes. Reaction for another 6h, precipitation w...
Embodiment 2
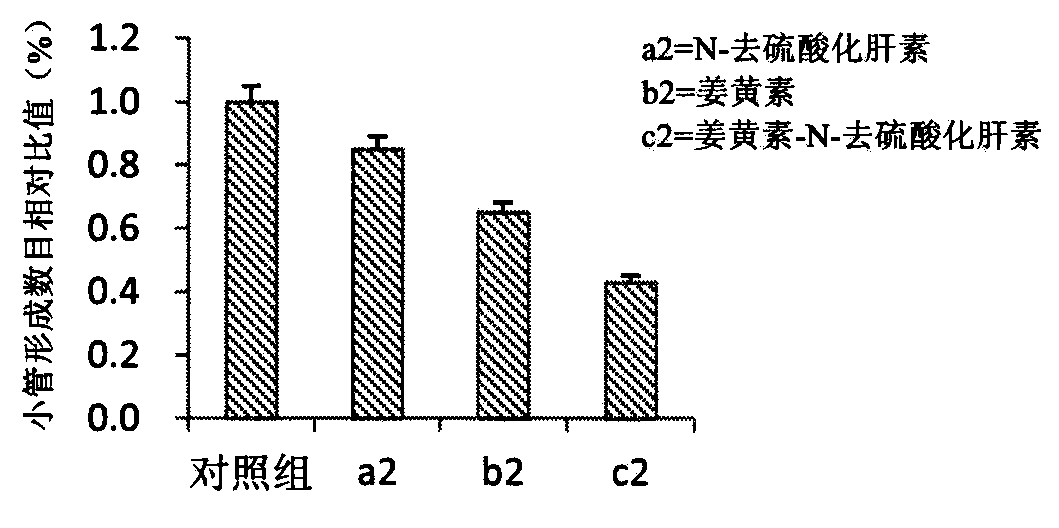
[0065] Example 2: Synthesis of Gambogic Acid-Cystamine-N-O-Desulfated Heparin Compound Drug
[0066] Weigh an appropriate amount of gambogic acid (GA) and put it in an eggplant-shaped bottle, add 30 mL of tetrahydrofuran, the molar concentration of GA is 0.02 mmol / mL, and under the conditions of dark light, ice bath, and inert gas protection, add dicyclohexylcarbon two in turn. The molar ratio of imine (DCC) and hydroxysuccinimide (NHS), GA, DCC and NHS was 1:1.5:1.2 in turn. After 30min reaction in ice bath, move to room temperature for 36h reaction. The precipitated dicyclohexylurea (DCU) was removed by vacuum filtration to obtain a filtrate. The filtrate was precipitated with 3 times the amount of n-hexane for 8 h, filtered and dried in vacuo to obtain succinimidyl GA. 0.20 g of succinimidyl GA was weighed, dissolved in 10 mL of N,N-dimethylformamide (DMF), and slowly added dropwise to 2 mL of cystamine for 45 minutes. Reaction for another 8h, saturated brine was precipi...
Embodiment 3
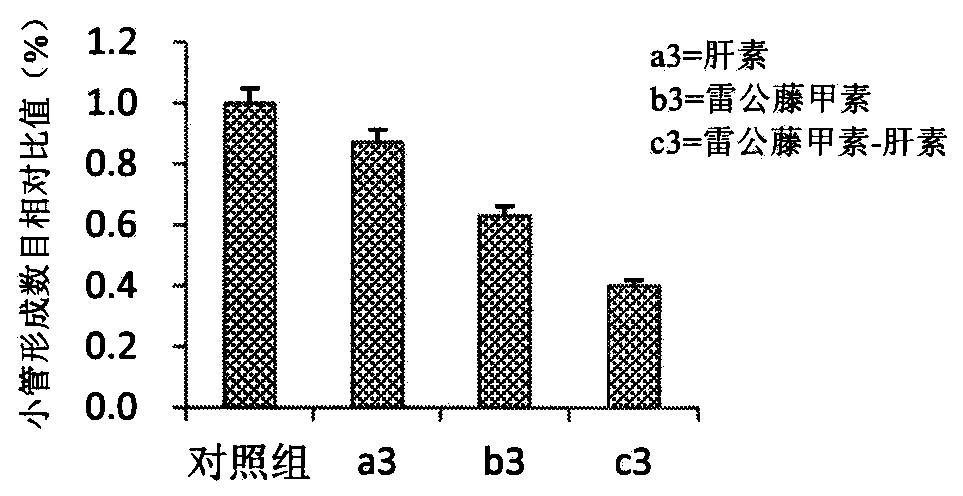
[0067] Example 3: Synthesis of rhein-p-phenylenediamine-low molecular weight heparin compound drug
[0068] Weigh an appropriate amount of rhein (Rh) and put it in an eggplant-shaped bottle, add 40 mL of tetrahydrofuran, the molar concentration of Rh is 0.1 mmol / mL, protect from light, ice bath, and under the protection of inert gas, add dicyclohexylcarbodiimide in turn. The molar ratio of amine (DCC) and hydroxysuccinimide (NHS), Rh, DCC and NHS is 1:1.2:1.2 in turn. The reaction was carried out in an ice bath for 60 min, and then moved to room temperature for 24 h. The precipitated dicyclohexylurea (DCU) was removed by vacuum filtration to obtain a filtrate. The filtrate was precipitated with 3 times the amount of n-hexane for 12 h, filtered, and dried in vacuo to obtain succinimidyl Rh. Weigh 0.50 g of succinimidyl Rh, dissolve it with 30 mL of N,N-dimethylformamide (DMF), and slowly add it dropwise to 2.5 mL of p-phenylenediamine for 80 minutes. Reaction for another 12h...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com