Curcumin prodrug micelle with oxidation and reduction sensitivity, micellar monomer and preparation method of micellar monomer
A technology of curcumin and micelles, which is applied in the field of pharmaceutical preparations, can solve the problems of poor water solubility of curcumin, achieve particle size and stability improvement, and enhance the effect of EPR
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
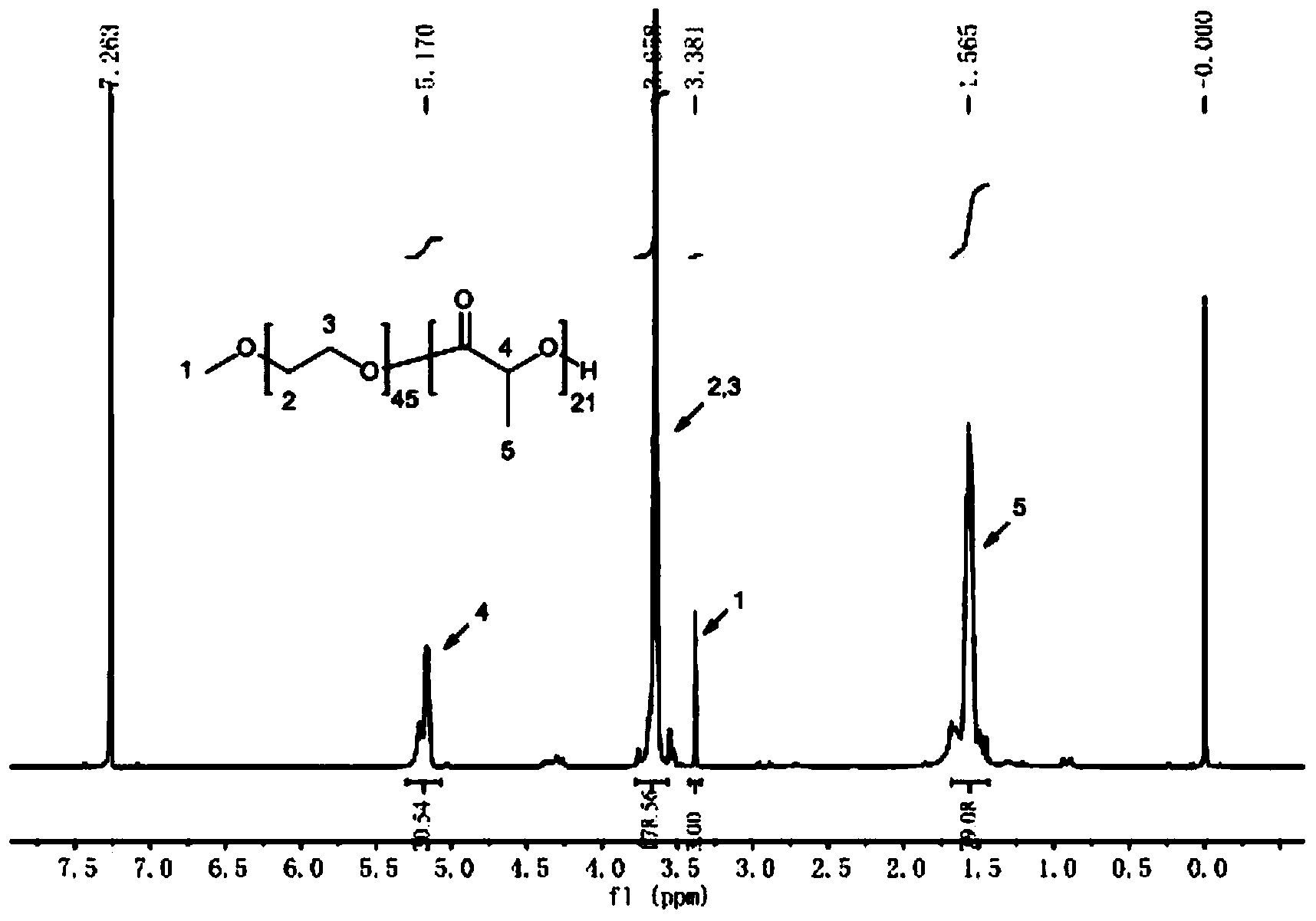
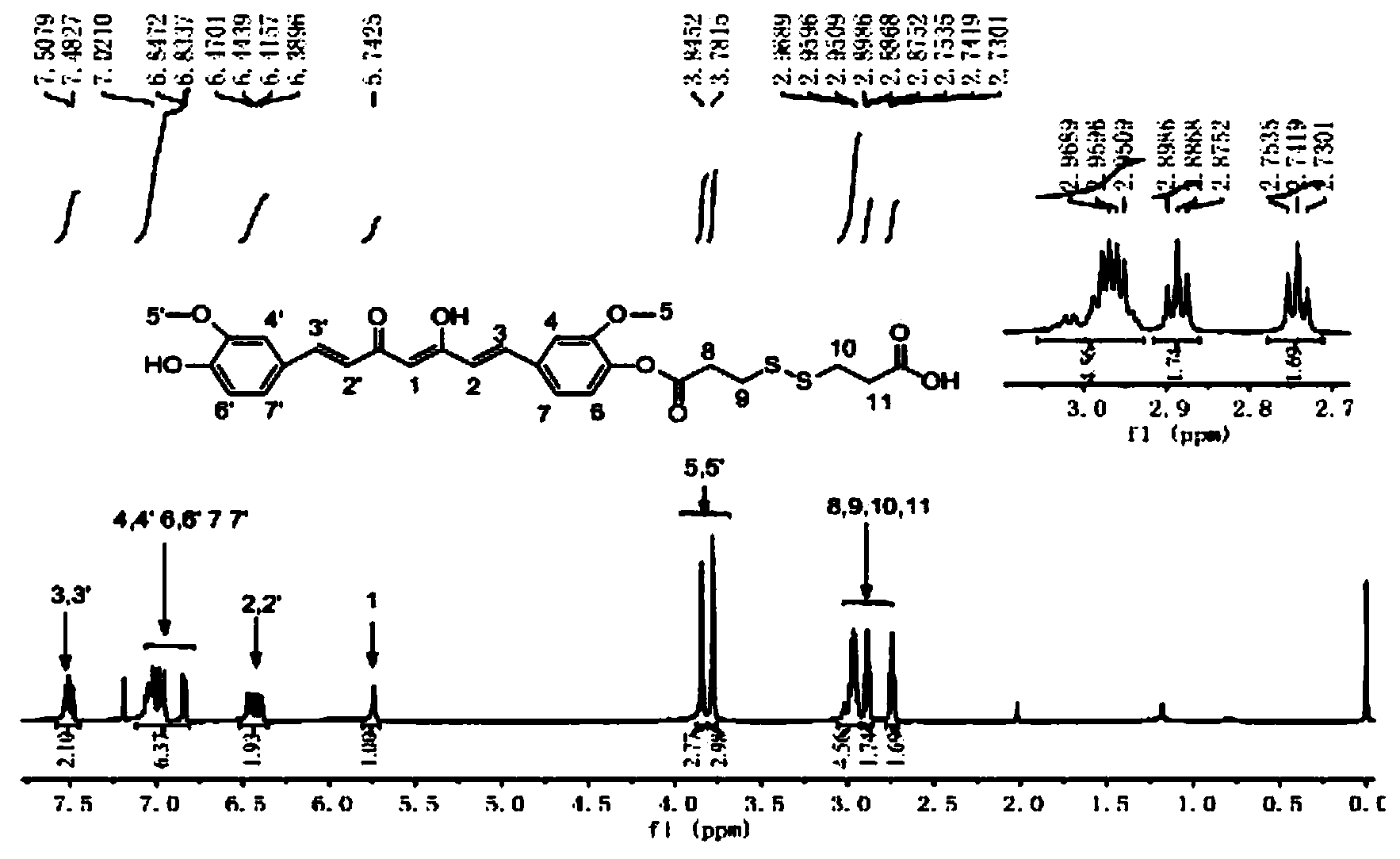
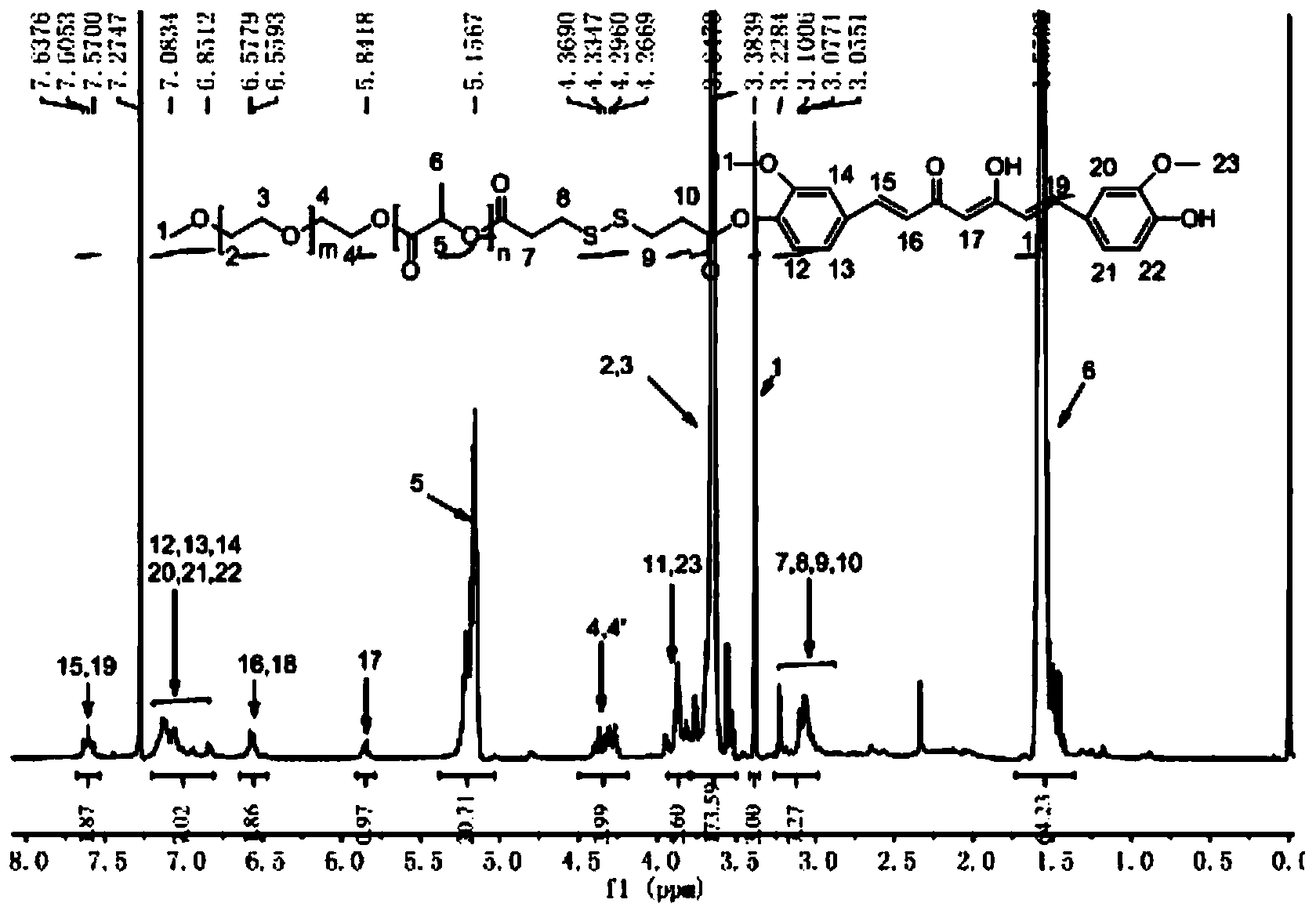
[0043] Example 1-1: Take 10g of MPEG2000 and place it in a 250mL round bottom flask, stir and melt in an oil bath at 60°C, then add 10g of lactide, heat up to 100°C until completely melted, vacuumize, protect with nitrogen, and add lactide The mass of 0.2% stannous octoate was heated to 125°C, and melted and polymerized for 24 hours. After the reaction is finished, wait for the system to cool down to room temperature, dissolve with an appropriate amount of tetrahydrofuran (using ultrasound), remove part of the solvent by rotary evaporation, drop into excess ice ether to precipitate, repeat three times, and filter with suction to obtain a white flocculent solid, which is dissolved in 15mL of tetrahydrofuran , put it into a dialysis bag with a molecular weight cut-off of 3500, and then put it into 1000mL distilled water for dialysis for 24 hours. Hour.
Embodiment 1-2
[0044] Example 1-2: Take 10g of MPEG2000 and place it in a 250mL round bottom flask, stir and melt in an oil bath at 60°C, then add 8g of lactide, heat up to 100°C until completely melted, vacuumize, protect with nitrogen, and add lactide The mass of 0.2% stannous octoate was heated to 125°C, and melted and polymerized for 24 hours. After the reaction is finished, wait for the system to cool down to room temperature, dissolve with an appropriate amount of tetrahydrofuran (using ultrasound), remove part of the solvent by rotary evaporation, drop into excess ice ether to precipitate, repeat three times, and filter with suction to obtain a white flocculent solid, which is dissolved in 15mL of tetrahydrofuran , put it into a dialysis bag with a molecular weight cut-off of 3500, and then put it into 1000mL distilled water for dialysis for 24 hours. Hour.
Embodiment 1-3
[0045] Example 1-3: Take 10g of MPEG2000 and place it in a 250mL round-bottomed flask, stir and melt in an oil bath at 60°C, then add 5g of lactide, heat up to 100°C until completely melted, vacuumize, protect with nitrogen, and add lactide The mass of 0.2% stannous octoate was heated to 125°C, and melted and polymerized for 24 hours. After the reaction is finished, wait for the system to cool down to room temperature, dissolve with an appropriate amount of tetrahydrofuran (using ultrasound), remove part of the solvent by rotary evaporation, drop into excess glacial ether to precipitate, repeat three times, and filter with suction to obtain a white flocculent solid, which is dissolved in 15mL of tetrahydrofuran , put it into a dialysis bag with a molecular weight cut-off of 3500, and then put it into 1000mL distilled water for dialysis for 24 hours. Hour.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com