Compound formulation for treating bacterial infection of digestive tract of livestock and poultry and preparation method of compound formulation
A compound preparation, digestive tract technology, applied in the directions of digestive system, antibacterial drugs, medical preparations containing active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
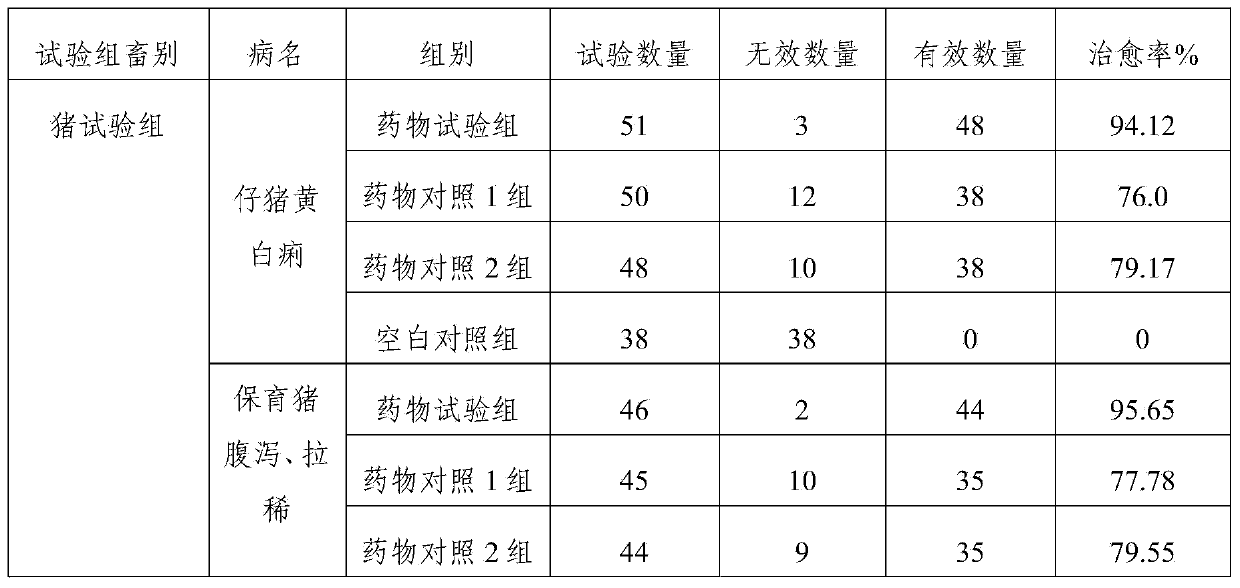
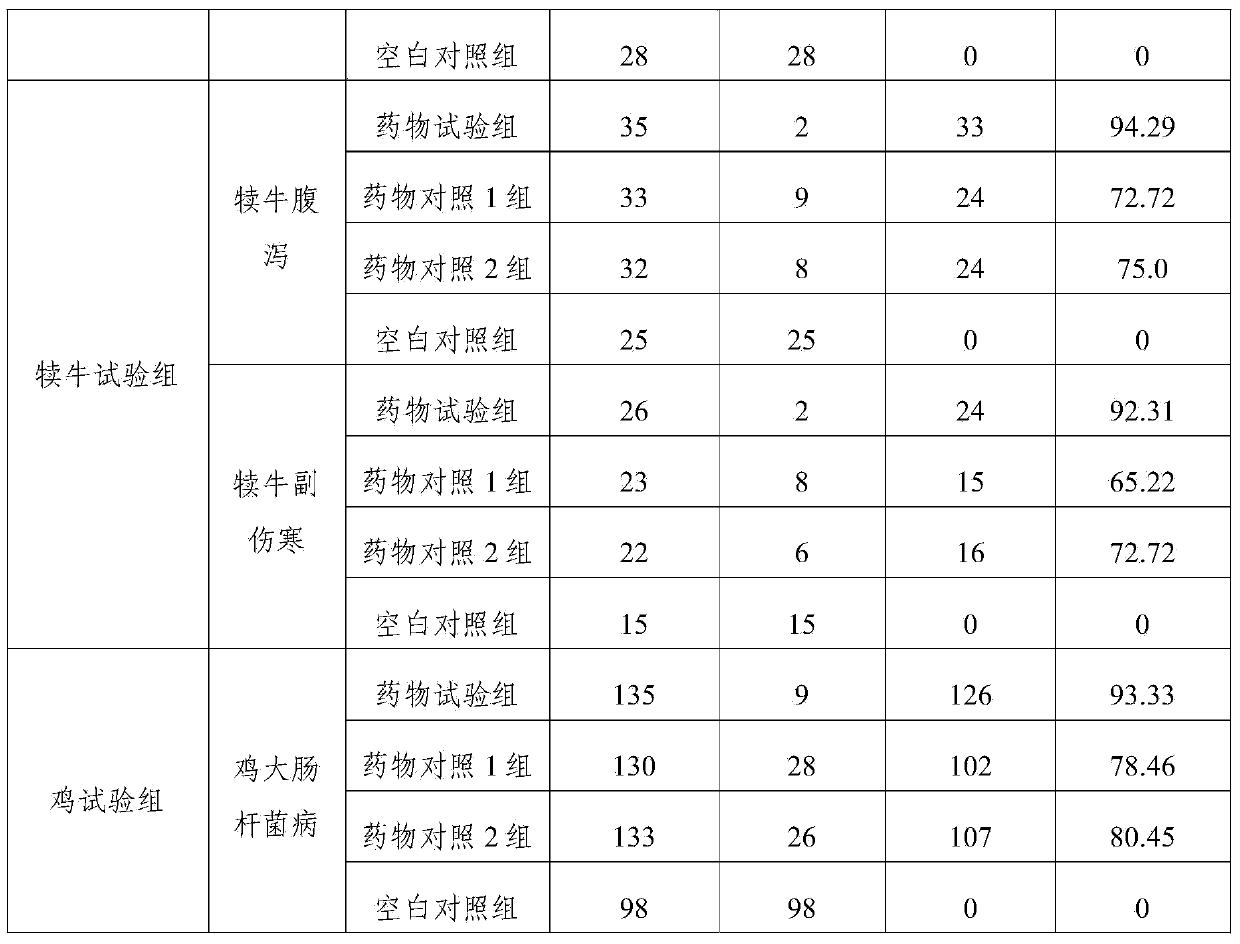
Examples
Embodiment 1
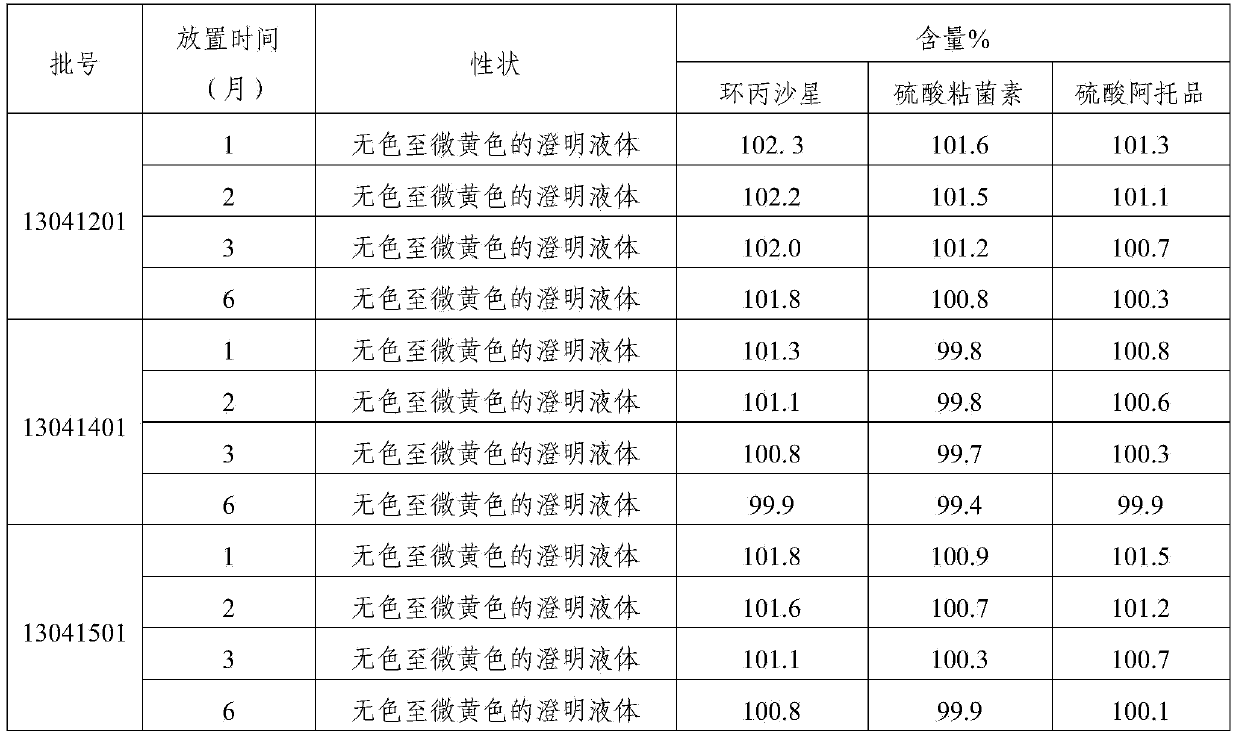
[0031] The compound preparation of this example contains the following components: ciprofloxacin lactate (calculated as ciprofloxacin) 5g, colistin sulfate (calculated as a pure drug) 4g, atropine sulfate 0.07g, procaine hydrochloride For 0.9g, 0.8g of sodium chloride, 5g of nicotinamide, 0.04g of edetate disodium, and appropriate amount of lactic acid, add water for injection to 100ml.
[0032] Described preparation method comprises the steps:
[0033] (1) Take 40% water for injection and heat it to 50°C, add colistin sulfate and stir to dissolve; then add pre-dissolved procaine hydrochloride and sodium chloride respectively, and fully stir to form A liquid;
[0034] (2) Take 30% water for injection, heat it to 60°C, put in ciprofloxacin lactate to dissolve, then put in pre-dissolved atropine sulfate and disodium edetate and stir to form B liquid;
[0035] (3) Combine liquid A and liquid B, add nicotinamide and stir evenly, add water for injection to 98%, adjust the pH value...
Embodiment 2
[0037] The compound preparation of this example contains the following components: ciprofloxacin lactate (calculated as ciprofloxacin) 2.5g, colistin sulfate (calculated as pure medicine) 2g, atropine sulfate 0.05g, profloxacin hydrochloride Caine 0.8g, sodium chloride 0.7g, nicotinamide 2.5g, edetate disodium 0.03g, lactic acid amount, water for injection was added to 100ml.
[0038] Described preparation method comprises the steps:
[0039] (1) Take 40% water for injection and heat it to 50°C, add colistin sulfate and stir to dissolve; then add pre-dissolved procaine hydrochloride and sodium chloride respectively, and fully stir to form A liquid;
[0040] (2) Take 30% water for injection, heat it to 60°C, put in ciprofloxacin lactate to dissolve, then put in pre-dissolved atropine sulfate and disodium edetate and stir to form B liquid;
[0041] (3) Combine liquid A and liquid B, add nicotinamide and stir evenly, add water for injection to 98%, adjust the pH value to 3.8-4.5...
Embodiment 3
[0043] The compound preparation of this example contains the following components: ciprofloxacin lactate (calculated as ciprofloxacin) 4g, colistin sulfate (calculated as pure medicine) 3g, atropine sulfate 0.05g, proca For 0.8g, 0.6g of sodium chloride, 4g of nicotinamide, 0.03g of edetate disodium, and appropriate amount of lactic acid, add water for injection to 100ml.
[0044] Described preparation method comprises the steps:
[0045] (1) Take 40% water for injection and heat it to 50°C, add colistin sulfate and stir to dissolve; then add pre-dissolved procaine hydrochloride and sodium chloride respectively, and fully stir to form A liquid;
[0046] (2) Take 30% water for injection, heat it to 60°C, put in ciprofloxacin lactate to dissolve, then put in pre-dissolved atropine sulfate and disodium edetate and stir to form B liquid;
[0047] (3) Combine liquid A and liquid B, add nicotinamide and stir evenly, add water for injection to 98%, adjust the pH value to 3.8-4.5 wit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com