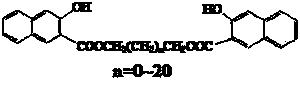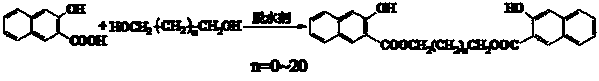3-hydroxyl-2-naphthoic acid (1,n-alkylene glycol) diester coupling agent and synthetic method thereof
A technology of hydroxyl-terminated straight-chain alkane diol and naphthoic acid is applied in the field of a new type of diazonium flake coupling agent - 3-hydroxy-2-naphthoic acid diester and its synthesis, which can solve the problem of aging and darkening in bright areas, light Density quality deterioration, color tone is not bright and other problems, to achieve the effect of improving photosensitive performance, photosensitive speed, easy to use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Add 3-hydroxy-2-naphthoic acid (10 g, 53 mmol), ethylene glycol (1.65 g, 26.5 mmol) into 55 mL toluene, about 1 mL trifluoromethanesulfonic acid (about 10 mmol), 110-120°C Reflux and split water for 5 h. After most of the solvent was evaporated, it was cooled and filtered. The filter cake was washed with hot sodium bicarbonate solution, washed with water until neutral, dried, mixed with tetrahydrofuran and toluene, and recrystallized to obtain 9.2 g of light yellow powder with a melting point of 172-175 ° C and a purity of more than 98 %, yield 86%.
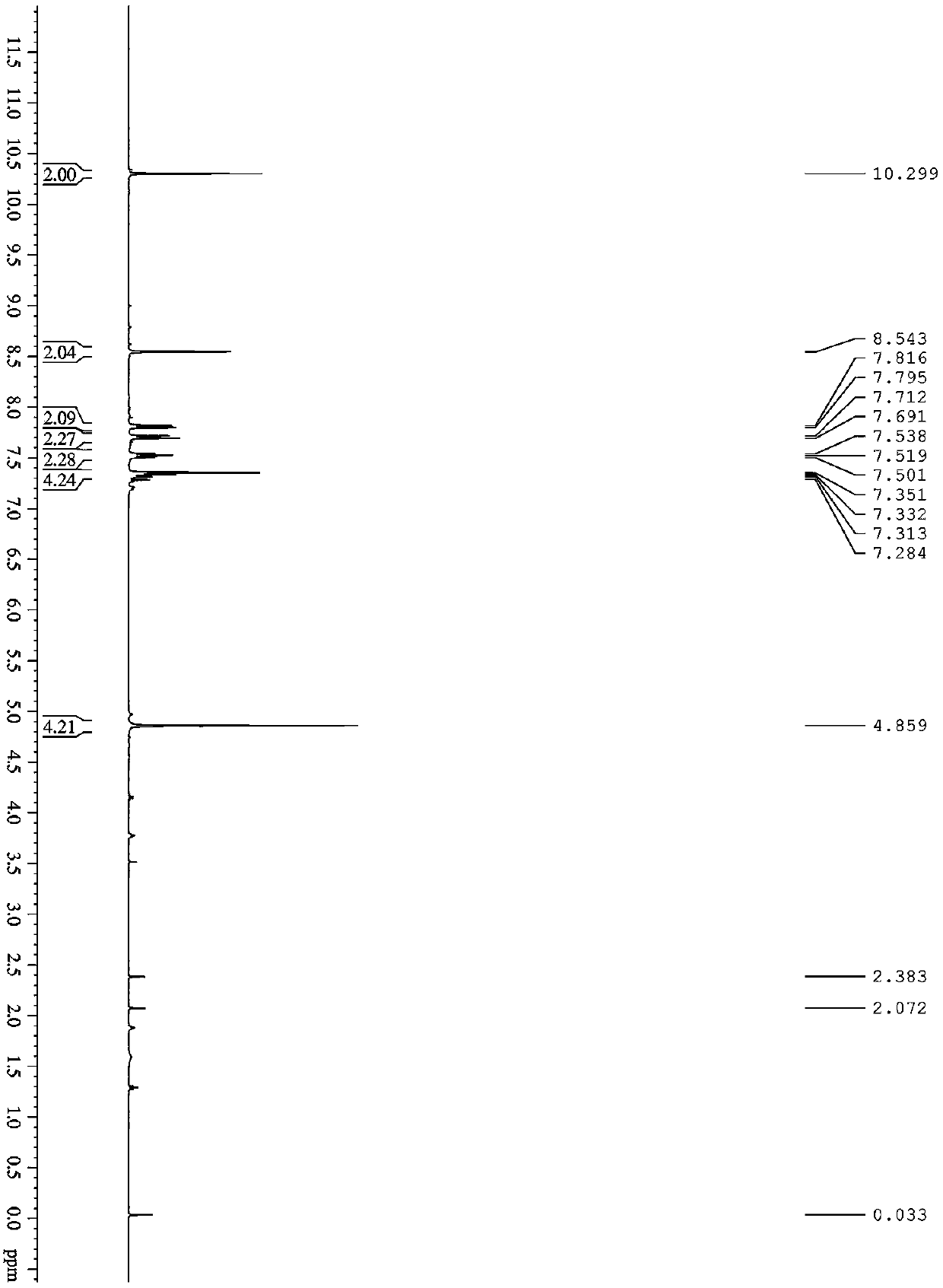
[0023] 1 H NMR (400 MHz, CDCl 3 ): δ (ppm) 10.30(s, 2H), 8.54 (s, 2H), 7.82–7.80 (m, 2H), 7.71-7.69 (m, 2H), 7.54-7.50 (m, 2H), 7.35-7.28 (m, 4H), 4.86 ppm (s, 4H); 13 C NMR (400 MHz, CDCl 3 ): δ (ppm) 164.21, 156.96, 139.83, 133.77, 129.92, 129.12, 128.17, 126.38, 124.74, 113.82, 111.74, 63.25. elemental analysis calcd (%) for C 24 h 18 o 6 : C 71.64, H 4.51; found: C 71.45, H 4.75. MS (EI, m / z) 402.41.
[0024]...
Embodiment 2
[0027] Add 3-hydroxy-2-naphthoic acid (10 g, 53 mmol), 1,3-propanediol (2.02 g, 26.5 mmol) into 55 mL of toluene, about 1 mL of trifluoromethanesulfonic acid (about 10 mmol), 110- Reflux at 120°C for 6 h. Evaporate most of the solvent, then cool and filter, wash the filter cake with hot sodium bicarbonate solution, wash with water until neutral, dry, mix and recrystallize with tetrahydrofuran and acetone to obtain 8.6 g of yellow powder, melting point 130-133 ° C, purity greater than 98% , yield 77.8%.
[0028] 1 H NMR (400 MHz, CDCl 3 ): δ (ppm) 10.30(s, 2H), 8.54 (s, 2H), 7.82–7.80 (m, 2H), 7.71-7.69 (m, 2H), 7.54-7.50 (m, 2H), 7.35-7.28 (m, 4H), 4.35-4.21 ppm (m, 4H), 2.41-2.37 ppm (m, 2H); 13 C NMR (400 MHz, CDCl 3 ): δ (ppm) 164.21, 156.96, 139.83, 133.77, 129.92, 129.12, 128.17, 126.38, 124.74, 113.82, 111.74, 61.15, 27.64. elemental analysis for calcd (%) 25 h 20 o 6 : C 72.10, H 4.84; found: C 72.13, H 4.81. MS (EI, m / z) 416.44.
[0029] Take 0.5 g 3-hydroxy-...
Embodiment 3
[0032] Add 3-hydroxy-2-naphthoic acid (10 g, 53 mmol), 1,4-butanediol (2.4 g, 26.5 mmol) into 40 mL of chlorobenzene, about 1 mL of p-toluenesulfonic acid (about 10 mmol), Reflux at 130-140°C and divide water for 7 hours. After most of the solvent was evaporated, it was cooled and filtered. The filter cake was washed with hot sodium bicarbonate solution, washed with water until neutral, dried, mixed with tetrahydrofuran, methanol and acetone, and recrystallized to obtain 7.4 g of bright yellow powder with a melting point of 152-154 °C and a purity of Greater than 98%, the yield is 64.7%.
[0033] 1 H NMR (400 MHz, CDCl 3 ): δ (ppm) 10.30(s, 2H), 8.54 (s, 2H), 7.82–7.80 (m, 2H), 7.71-7.69 (m, 2H), 7.54-7.50 (m, 2H), 7.35-7.28 (m, 4H), 4.35-4.21 ppm (m, 4H), 1.86-1.83 ppm (m, 4H); 13 C NMR (400 MHz, CDCl 3 ): δ (ppm) 164.21, 156.96, 139.83, 133.77, 129.92, 129.12, 128.17, 126.38, 124.74, 113.82, 111.74, 64.55, 25.14. elemental analysis for calcd (%) 26 h 22 o 6 : C 72.5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com