Multi-cell ranolazine slow-release pellet tablet
A technology of sustained-release pellets and sustained-release pellets, which is applied in the fields of pill delivery, medical preparations of non-active ingredients, cardiovascular system diseases, etc., and can solve problems such as pellet rupture, adhesion, and impact on formability, and achieve High bioavailability, large distribution area, good clinical significance effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
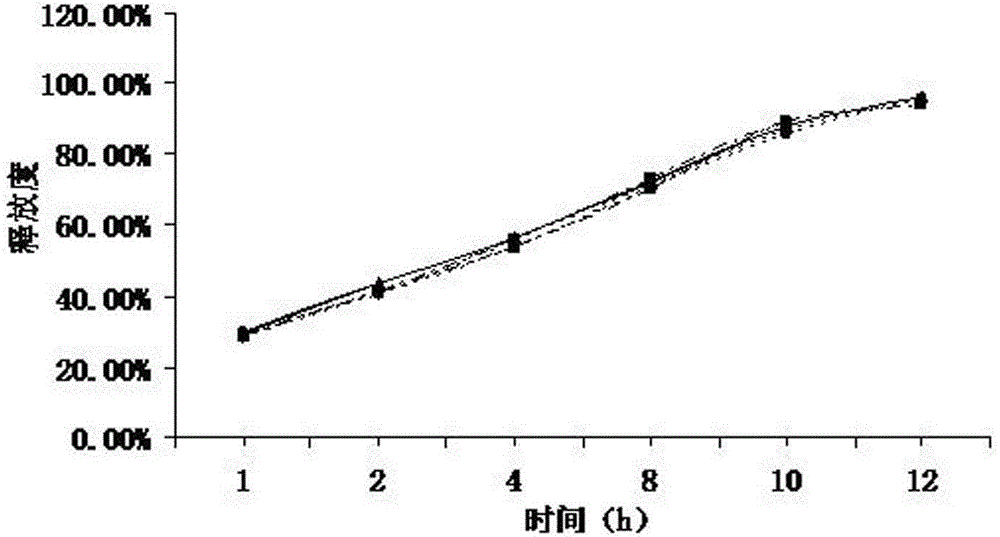
[0040]Weigh 60g of ranolazine, 25g of microcrystalline cellulose, 7g of lactose, 7g of precrossified starch, 1g of sodium lauryl sulfate, pass through a 100-mesh sieve and mix evenly, and use a 10% aqueous solution of povidone K30 as a binder to prepare Soft material, extruded and spheronized to prepare ranolazine drug-containing pellets, wherein the extrusion screen has an aperture of 0.6 mm, an extrusion speed of 25 r / min, dried at 40°C in a fluidized bed for 1 hour, and sieved to 20-40 mesh for use.
[0041] Put the screened ranolazine pellets in a fluidized bed, prepare a coating solution, adjust the fan frequency to 25HZ, the air inlet temperature to 30°C, the material temperature to 25°C and the flow rate of the coating solution to 0.4rpm, and control the coating weight gain to 5%. Ranolazine sustained-release pellets were prepared.
[0042] Coating solution ratio:
[0043] Sustained release material Acrylic resin 15%
[0044] Plasticizer Diethyl phthalate 3%
[0045]...
Embodiment 2
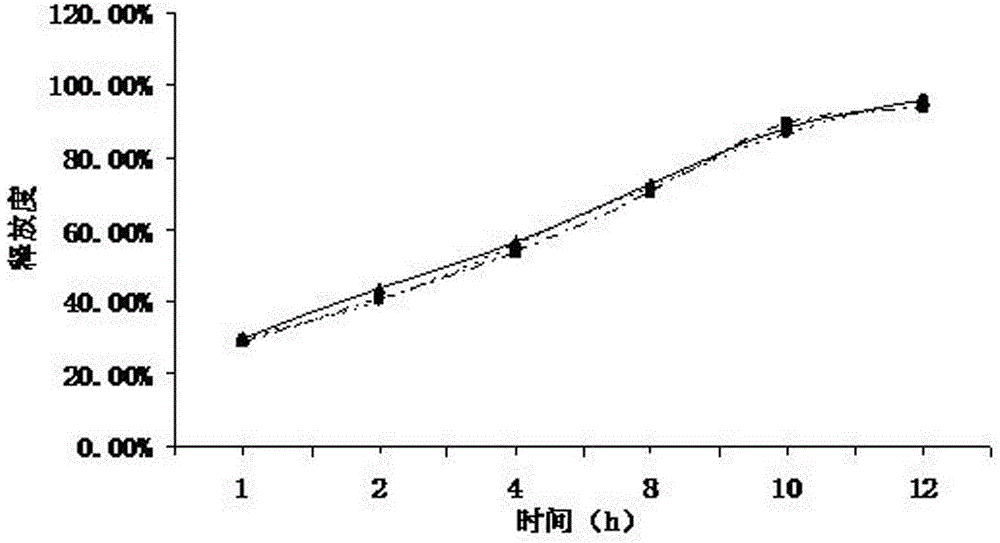
[0049] Weigh 80g of ranolazine, 15g of microcrystalline cellulose, 2g of lactose, 2g of starch, 0.8g of sodium lauryl sulfate, pass through a 100-mesh sieve and mix evenly, use 5% hypromellose E50M aqueous solution as a binder to make soft material, extruded and spheronized to prepare ranolazine drug-containing pellets, wherein the extrusion screen has an aperture of 0.8 mm, an extrusion speed of 25 r / min, dried at 40° C. in a fluidized bed for 1 hour, and sieved to 20-40 meshes for use.
[0050] The screened ranolazine pellets are placed in a fluidized bed to prepare a coating solution. Adjust the fan frequency to 25HZ, the air inlet temperature to 30°C, the material temperature to 25°C, and the flow rate of the coating solution to 0.4rpm, and control the coating weight gain to 5%, to prepare ranolazine sustained-release pellets.
[0051] Coating solution ratio:
[0052] Sustained release material Hypromellose 40%
[0053] Plasticizer triethyl citrate 5%
[0054] Anti-stic...
Embodiment 3
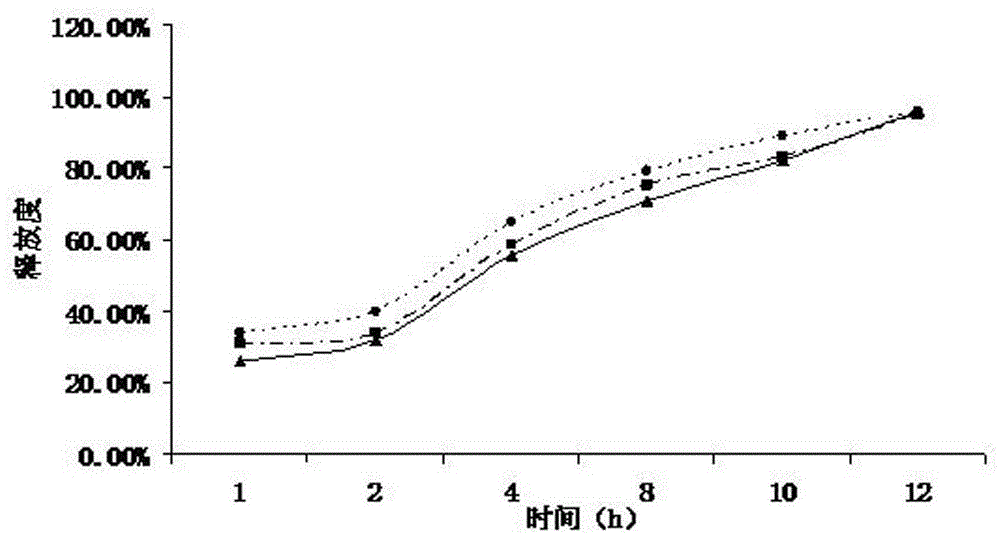
[0059] Weigh 95g of ranolazine, 3g of microcrystalline cellulose, 1g of lactose, and 1g of sodium lauryl sulfate, pass through a 100-mesh sieve and mix evenly, use 5% hypromellose K100M aqueous solution as a binder to make a soft material, and extrude Prepare ranolazine drug-containing pellets by spheronizing, wherein the extrusion screen has an aperture of 0.6mm, an extrusion speed of 25r / min, and is dried in a fluidized bed at 40°C for 1 hour, and sieved to 20-40 mesh for use.
[0060] The screened ranolazine pellets are placed in a fluidized bed to prepare a coating solution. Adjust the fan frequency to 25HZ, the air inlet temperature to 30°C, the material temperature to 25°C, and the flow rate of the coating solution to 0.4rpm, and control the coating weight gain to 5%, to prepare ranolazine sustained-release pellets.
[0061] Coating solution ratio:
[0062] Sustained release material Polyethylene glycol 5%
[0063] Plasticizers Diethyl phthalate, Dimethyl phthalate 6% ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com