Cationic polyamino acid group carrier material and preparation method thereof
A technology of polyamino acid and gene carrier, applied in the field of cationic polyamino acid gene carrier material and preparation thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
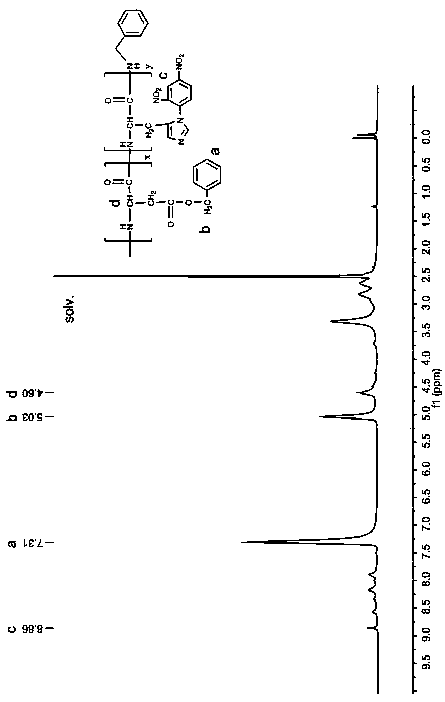
[0027] (1) Preparation of random copolymer of histidine and aspartic acid:
[0028] Weigh 1g N im-CBZ-N-DNP-L-histidine was dissolved in 10ml of tetrahydrofuran, and under the protection of nitrogen, 5ml of tetrahydrofuran solution containing 0.174ml of thionyl chloride was added dropwise, and reacted at room temperature for 20 minutes. After the solution became clear, it was immediately poured into 80ml of A pale yellow precipitate was obtained in water ether, and the filter residue was vacuum-dried to obtain histidine NCA (HIS-NCA), which was washed twice with nitromethane and ether to obtain pure histidine NCA.
[0029] Weigh 0.9g β-benzyl L-aspartate acid and dissolve it in 10ml tetrahydrofuran, then weigh 0.47g triphosgene and dissolve it in 5ml tetrahydrofuran, under the protection of nitrogen, drop triphosgene into the benzyl aspartate solution and react at 50°C After 30 minutes, after the solution became clear, it was immediately poured into 80ml of n-hexane ...
Embodiment 2
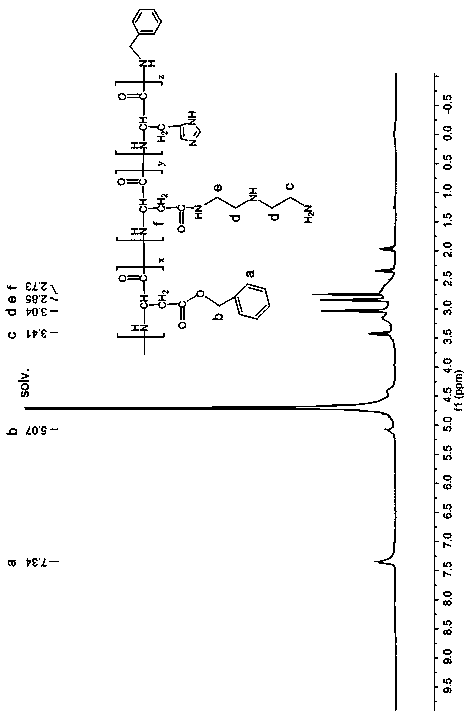
[0036] (1) Preparation of random copolymer of histidine and aspartic acid:
[0037] Weigh 0.5g N im -CBZ-N-DNP-L-histidine was dissolved in 8ml of tetrahydrofuran, under the protection of nitrogen, 4ml of tetrahydrofuran solution containing 0.09ml of thionyl chloride was added dropwise, and reacted at room temperature for 20 minutes. After the solution became clear, it was immediately poured into 64ml of A pale yellow precipitate was obtained in water ether, and the filter residue was vacuum-dried to obtain histidine NCA (HIS-NCA), which was washed twice with nitromethane and ether to obtain pure histidine NCA.
[0038] Weigh 2.7g β-benzyl L-aspartate acid and dissolve it in 20ml tetrahydrofuran, then weigh 1.41g triphosgene and dissolve it in 10ml tetrahydrofuran. Under the protection of nitrogen, drop triphosgene into the benzyl aspartate solution and react at 40°C After 40 minutes, after the solution became clear, it was immediately poured into 160ml of n-hexane...
Embodiment 3
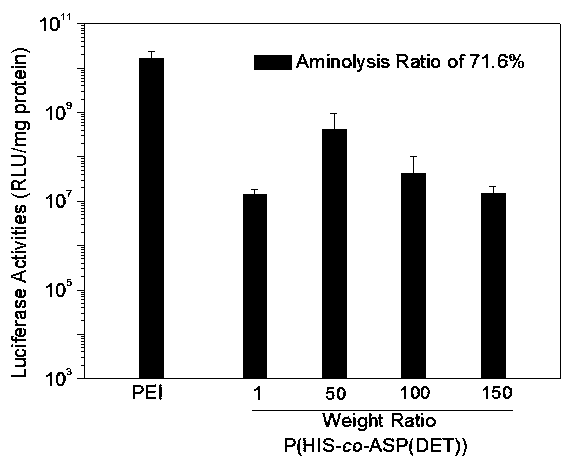
[0045] (1) Preparation of random copolymer of histidine and aspartic acid:
[0046] Weigh 1g N im -CBZ-N-DNP-L-histidine was dissolved in 10ml of tetrahydrofuran, and under the protection of nitrogen, 5ml of tetrahydrofuran solution containing 0.174ml of thionyl chloride was added dropwise, and reacted at room temperature for 20 minutes. After the solution became clear, it was immediately poured into 100ml of A pale yellow precipitate was obtained in water ether, and the filter residue was vacuum-dried to obtain histidine NCA (HIS-NCA), which was washed twice with nitromethane and ether to obtain pure histidine NCA.
[0047] Weigh 0.9g β-benzyl L-aspartate acid and dissolve it in 10ml tetrahydrofuran, then weigh 0.51g triphosgene and dissolve it in 5ml tetrahydrofuran, under the protection of nitrogen, drop triphosgene into the benzyl aspartate solution and react at 50°C After 30 minutes, after the solution became clear, it was immediately poured into 100ml of n-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com