Molecular probe for detecting cyanide ions and synthesis and application method thereof
A molecular probe and cyanide ion technology, applied in the field of chemical analysis and detection, can solve the problems of complex and time-consuming processing, and achieve the effects of stable optical performance, fast response speed and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Example 1: Synthesis of Colorimetric Molecular Probes
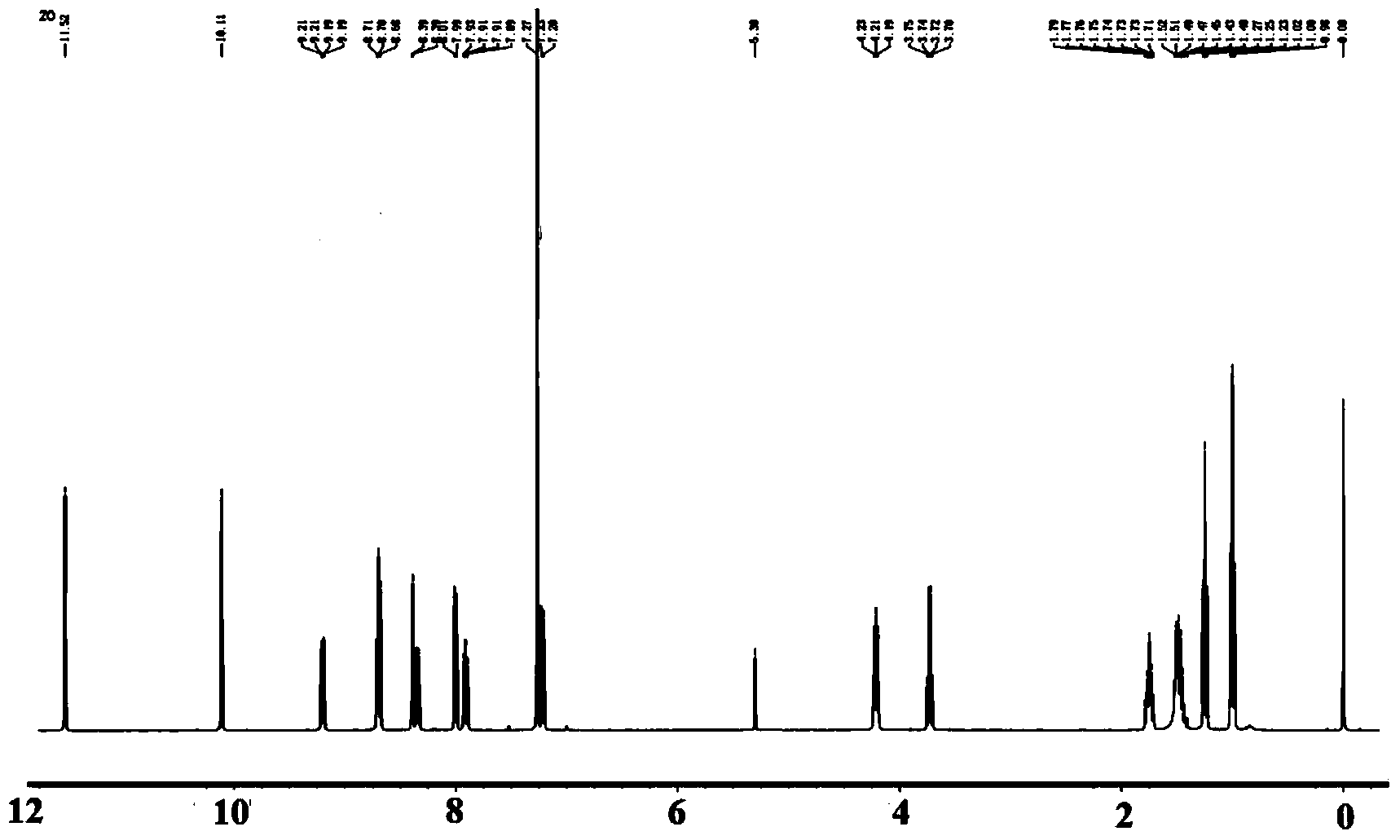
[0053] Sodium nitrite (70 mg, 1 mmol) was slowly added to concentrated sulfuric acid (1.5 mL, 98%) in an ice bath for 10 min, and stirring was continued for 10 min. The solution was heated to 60°C and stirred until all the solids were dissolved. Then put the reaction in an ice bath, add N-butyl-4-amino-1,8-naphthalimide (0.27g, 1mmol) to the reaction solution in 30min, then add 1mL acetic acid, and continue the ice bath reaction After 4 hours. A methanol (30 mL) solution containing salicylaldehyde (80 mg, 1.1 mmol) was dropped into the above reaction solution, and the pH of the reaction solution was adjusted to 5 with saturated sodium acetate solution, and then the reaction was moved to room temperature for 3 h. After the reaction was completed, the solid was filtered and washed three times with hot water, then added dichloromethane to dissolve and dried with anhydrous sodium sulfate, the solvent was distilled of...
Embodiment 2
[0056] Embodiment 2: Probe detects cyanide ion in naked eye and colorimetric method
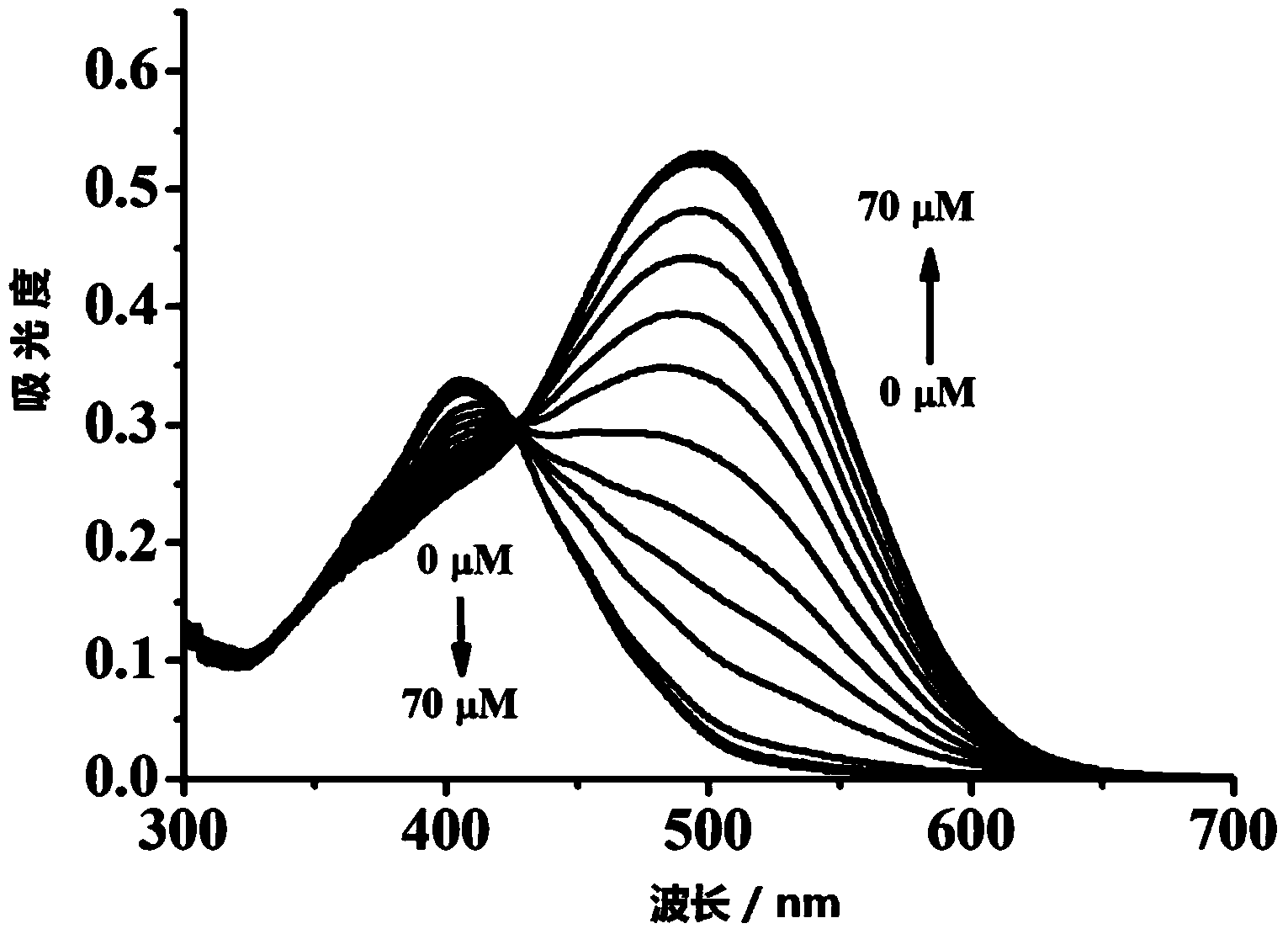
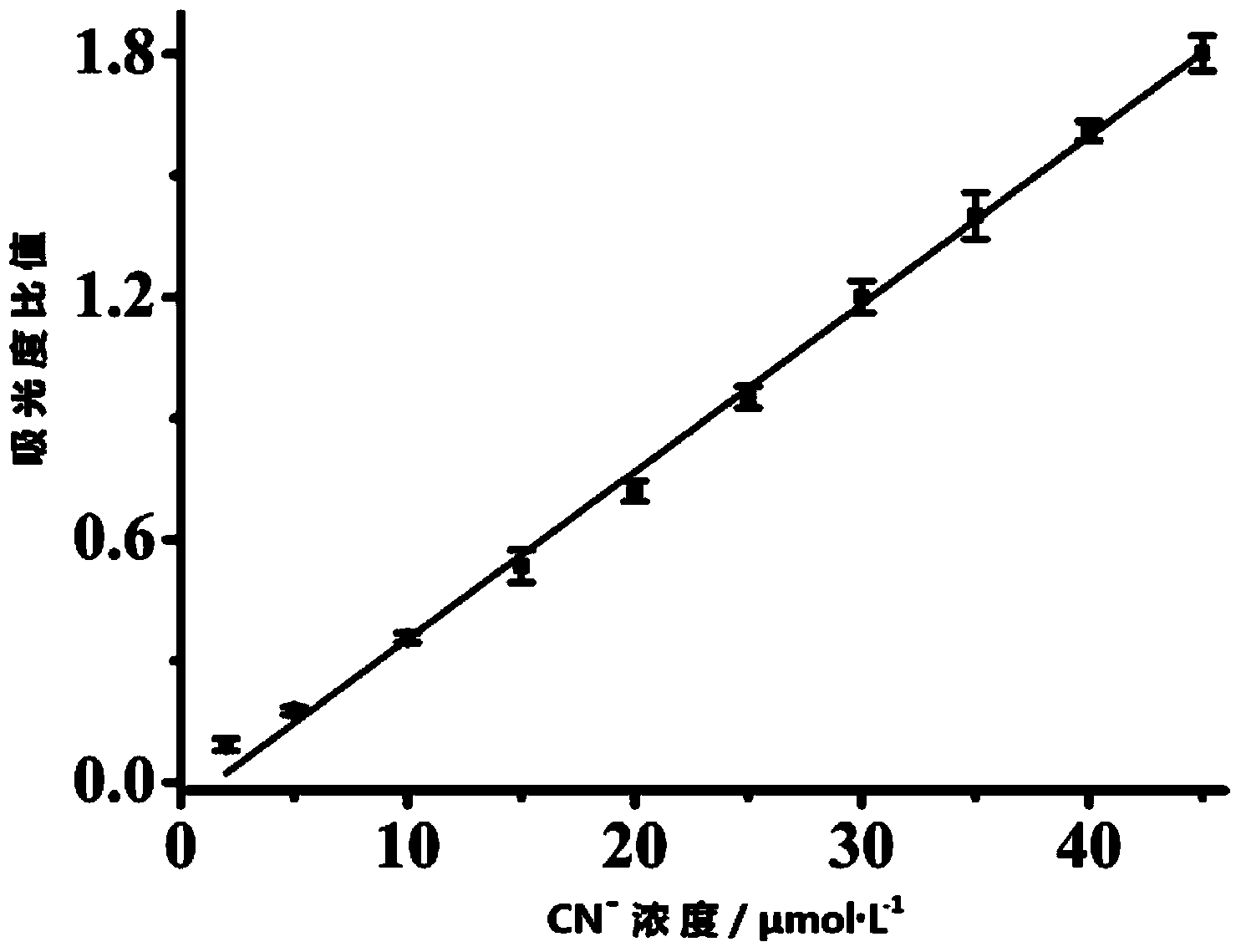
[0057] Dissolve the colorimetric molecular probe prepared above in H 2 O: DMSO in a mixed solvent of 7:3, prepared to 25 μmol L -1 probe solution. Add 2 mL of prepared 25 μmol L to a 3 mL cuvette -1 Probe solution, and then add different concentrations of CN - (Tetrabutylammonium salt) after mixing evenly, test its ultraviolet spectrum, the result is as follows figure 2 As shown,. CN - Concentration Mapping, CN - Concentration at 2–45μmol·L -1 In the range, there is a good linear relationship between the two ( image 3 ), can realize CN within the concentration range - Quantitative detection, and the color of the solution changes significantly, it is also suitable for naked eye detection. And this probe is not affected by some other common anions, such as: AcO - ,F - , Cl - ,Br - , I - ,N 3 - ,S 2- ,SCN - ,NO 3 - ,H 2 PO 3 - . Under the conditions that the above-ment...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com