Preparation method of vitamin E acetate
A technology of acetate and vitamin, applied in the field of preparation of vitamin E acetate, can solve the problems of large energy consumption, influence yield and quality, low conversion rate, etc., to reduce reaction by-products, improve product yield and by-products less effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
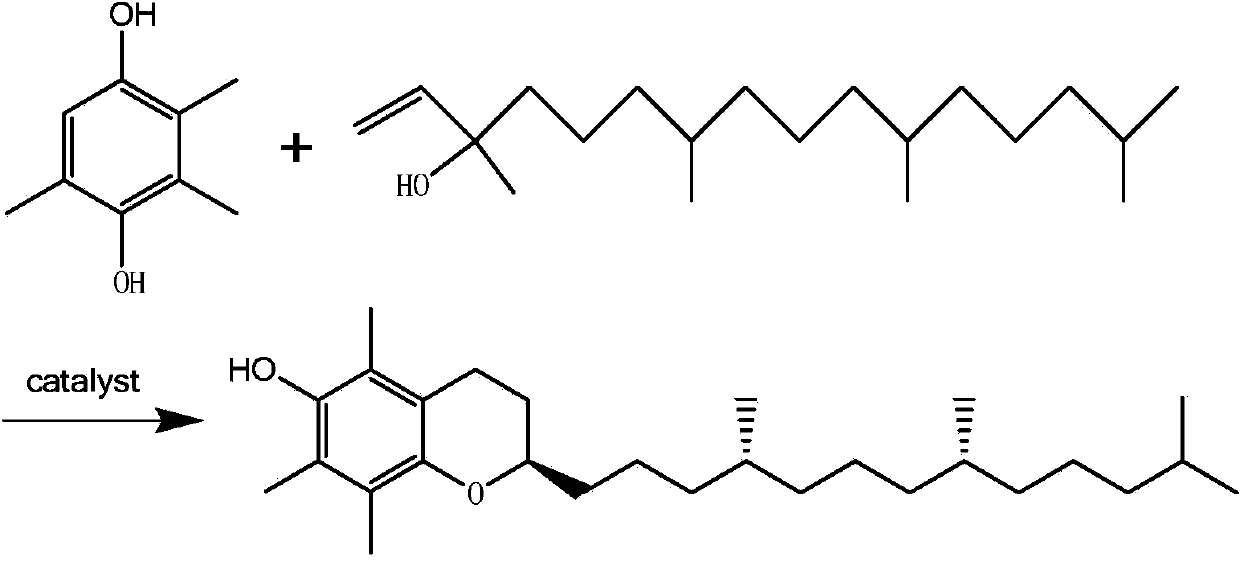
[0025] Add 600g of n-pentane and 300g of 2,3,5-trimethylhydroquinone to a 2L four-neck flask equipped with a stirrer, thermometer, oil-water separator, condenser tube, nitrogen inlet and isobaric titration funnel , 6g of n-tridecylamine and 200g of zinc chloride, add 5mL of hydrochloric acid with a mass fraction of 31%, stir and heat up to 36°C under nitrogen protection, and drop in 605g of isophytic alcohol at a constant pressure for 2 hours, drop After adding, keep warm for 1 hour, add 100mL of water to wash, add n-butanol to the washing liquid, extract, and then separate the liquid through the oil-water separator. The aqueous phase containing zinc chloride is reserved for the next step, and the organic phase is distilled to remove the solvent. Then add 200g of acetic anhydride, carry out esterification reaction at 140°C for 3 hours under the protection of nitrogen, recover the unreacted acetic anhydride, wash with water and distill at 140°C under normal pressure, the bottom ...
Embodiment 2
[0027] In a 2L four-neck flask equipped with a stirrer, a thermometer, an oil-water separator, a condensing tube and a nitrogen inlet isobaric titration funnel, add 600g of n-hexane, 300g of trimethylhydroquinone, 6g of n-tridecylamine, For the aqueous phase containing zinc chloride in the above example 1, add 40 g of zinc chloride, add 5 mL of hydrobromic acid (mass fraction 30%), stir and heat up to 98 ° C under the protection of nitrogen, and reflux within 2.5 hours. Add 605g of isophytic alcohol dropwise at a constant speed, while adding dropwise, use the oil-water separator to recover the water in the mixed solution. After the dropwise addition is completed, reflux and keep warm for 1 hour, add 100mL of water for washing, and add n-butanol to the washing solution. Extraction, and then liquid separation by oil-water separator, the water phase is a colorless solution, the oil phase is washed with water, and the water phase is combined twice for the next step, the organic pha...
Embodiment 3
[0029] Add 600g of n-heptane, 300g of trimethylhydroquinone, and 6g of n-tridecylamine to a 2L four-neck flask equipped with a stirrer, a thermometer, an oil-water separator, a condenser tube, and an isobaric titration funnel at the nitrogen inlet. Combined with the aqueous phase containing zinc chloride in Example 2 above, add 20g of zinc chloride, add 5mL of hydrobromic acid (mass fraction 30%), stir and heat up to 98°C under nitrogen protection, and reflux at 2.5 Add 605g of isophytic alcohol dropwise at a constant pressure and at a constant speed for 1 hour, and recover water while adding dropwise. Separation device, the aqueous phase containing zinc chloride is reserved for the next step, the organic phase is distilled to remove the solvent, then 200g of acetic anhydride is added, and the esterification reaction is carried out at 140°C for 3 hours under the protection of nitrogen, the unreacted acetic anhydride is recovered, washed with water and Atmospheric pressure dist...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com