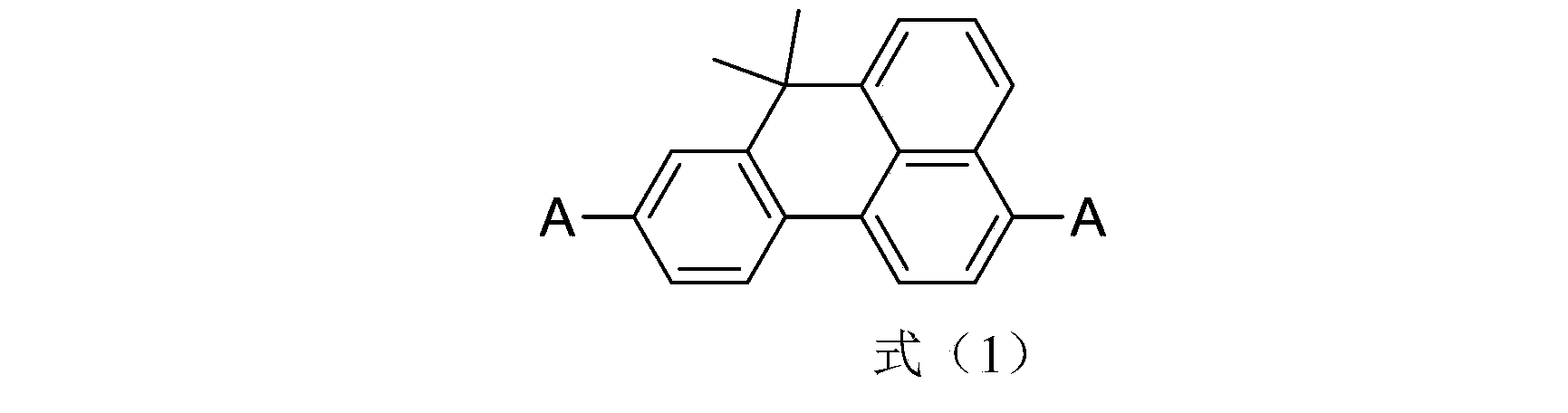
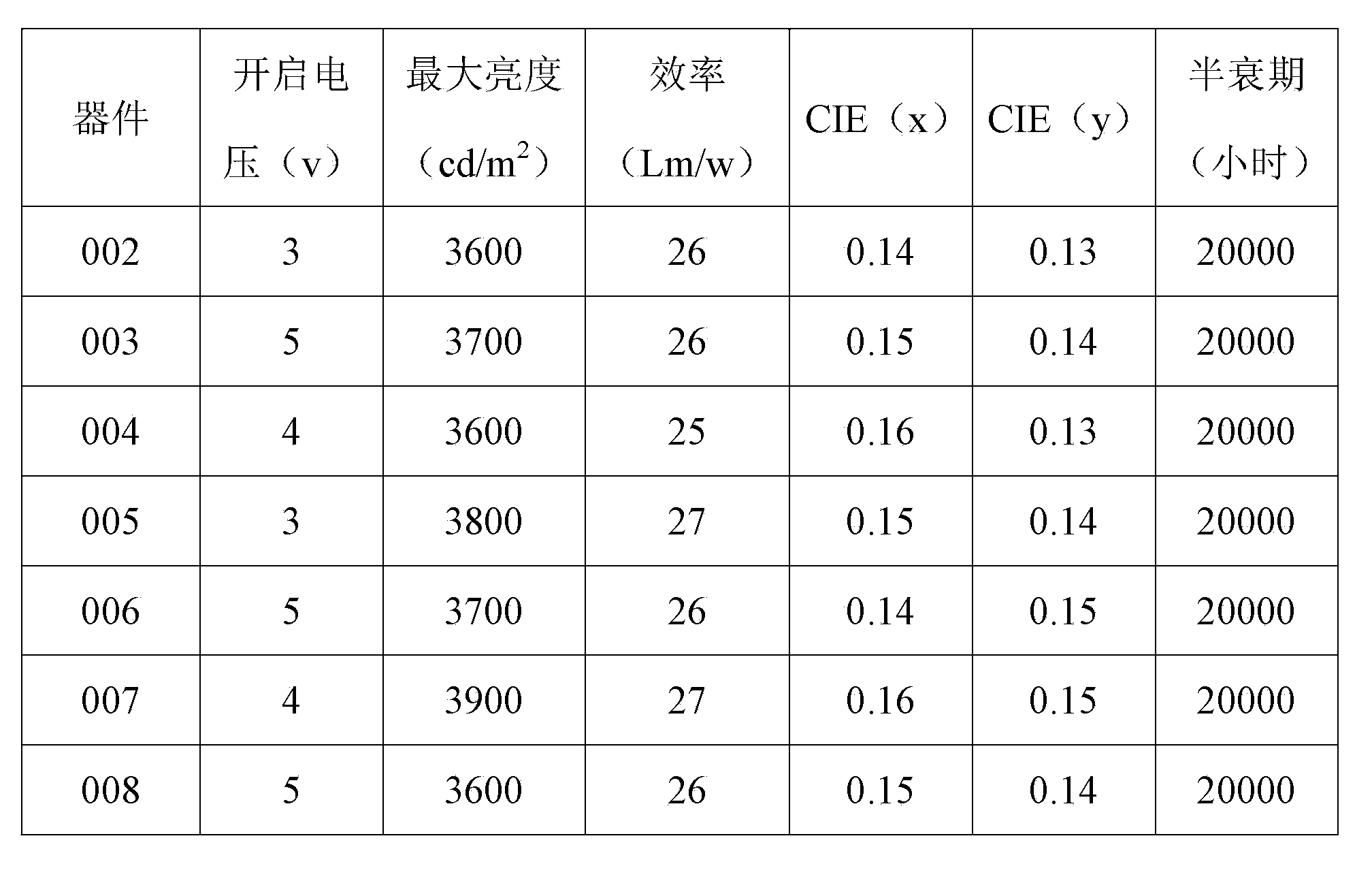
Benzanthracene organic luminescent material, and preparation method and application thereof
A technology of luminescent materials and benzanthracene, which is applied in the fields of luminescent materials, organic chemistry, chemical instruments and methods, etc., can solve the problems of low cost and the inability of blue light materials to meet industrial production, and achieve easy processing, improved luminous efficiency, and film formation good performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
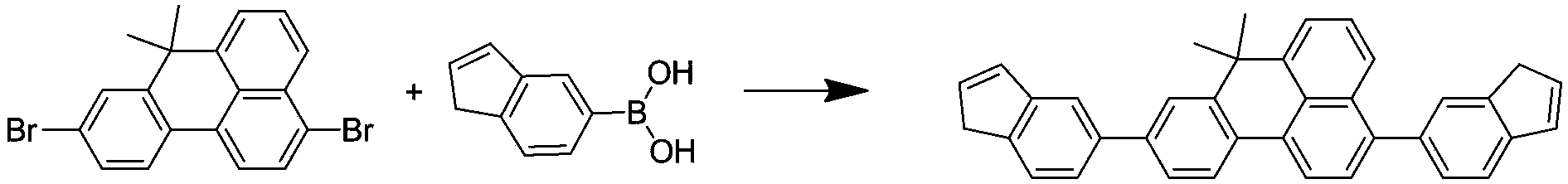
[0030] Embodiment 1: the synthesis of compound 001
[0031] The specific synthetic route is shown in the following formula:
[0032] Add 18.18g of 3,9-dibromo-7,7-dimethyl-7H-benzoanthracene, 11.30g of N-phenyl-2-carbazolylboronic acid, 20g of sodium carbonate, 250ml of tetrahydrofuran and 125ml of water into the three-necked flask , degassing, add 0.9g tetrakis(triphenylphosphine)palladium, heat up to 90°C, reflux for 24 hours, cool to room temperature, after the solid precipitates, suction filter, the filter cake is washed with water, ethanol and ether, and dried 30.32 g of 3,9-bis(N-phenylcarbazolyl)-7,7-dimethyl-7H-benzanthracene was obtained, with a yield of over 92% and an HPLC purity of over 98%. Mass Spectrum: Calculated 728.92; Found 728.90. Elemental analysis: Calculated for C: 90.63%; H: 5.53%; N: 3.84%; tested for C: 90.62%; H: 5.54%; N: 3.84%.
Embodiment 2
[0033] Embodiment 2: the synthesis of compound 002
[0034] The specific synthetic route is shown in the following formula:
[0035]
[0036] Add 18.18g of 3,9-dibromo-7,7-dimethyl-7H-benzoanthracene, 15.63g of 3-phenanthroline boronic acid, 20g of sodium carbonate, 250ml of tetrahydrofuran and 125ml of water into a three-necked flask, degas, Add 0.9g of tetrakis(triphenylphosphine)palladium, raise the temperature to 100°C, reflux for 27 hours, cool to room temperature, after the solid precipitates, filter with suction, wash the filter cake with water, ethanol and ether, and dry to obtain 3,9 - 25.26 g of bis(phenanthrolinyl)-7,7-dimethyl-7H-benzanthracene, the yield is over 93%, and the HPLC purity is over 98%. Mass Spectrum: Calcd. 600.71; Tested 600.69. Elemental analysis: calculated value C: 85.98%; H: 4.70%; N: 9.33%; tested value C: 85.96%; H: 4.72%; N: 9.32%.
Embodiment 3
[0037] Embodiment 3: the synthesis of compound 003
[0038] The specific synthetic route is shown in the following formula:
[0039]
[0040] Add 18.18g of 3,9-dibromo-7,7-dimethyl-7H-benzoanthracene, 11.30g of 5-benzofuranylboronic acid, 20g of sodium carbonate, 250ml of tetrahydrofuran and 125ml of water into a three-necked flask, degas, Add 0.9g of tetrakis(triphenylphosphine)palladium, raise the temperature to 100°C, reflux for 30 hours, cool to room temperature, after the solid precipitates, filter with suction, wash the filter cake with water, ethanol and ether, and dry to obtain 3,9 -Bis(benzofuryl)-7,7-dimethyl-7H-benzanthracene 19.40g, the yield is over 90%, and the HPLC purity is over 98%. Mass Spectrum: Calculated 476.56; Found 476.54. Elemental analysis: Calculated for C: 88.21%; H: 5.08%; O: 6.71%; tested for C: 88.20%; H: 5.07%; O: 6.72%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com