Chronic obstructive pulmonary disease treatment drug composition, preparation method and application thereof
A technology for chronic obstructive pulmonary disease and composition, which is applied in the directions of drug combination, pharmaceutical formula, respiratory system diseases, etc., can solve the problem of chronic obstructive pulmonary disease with unclear effective parts and active ingredients, and no retrieval of aster powder or aster and aster. The effective parts of coltsfoot flower, inconvenience to carry and use, etc., achieve the effect of improving clinical effectiveness and safety, improving quality of life, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
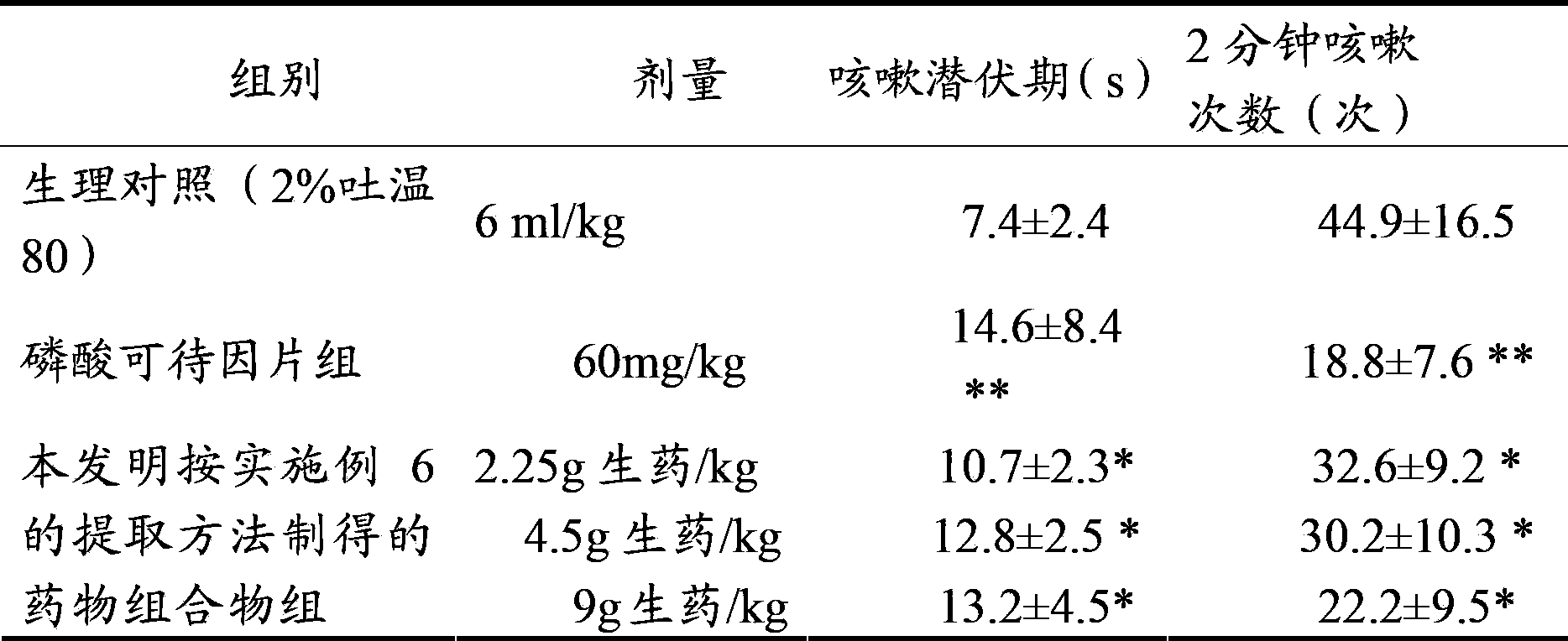
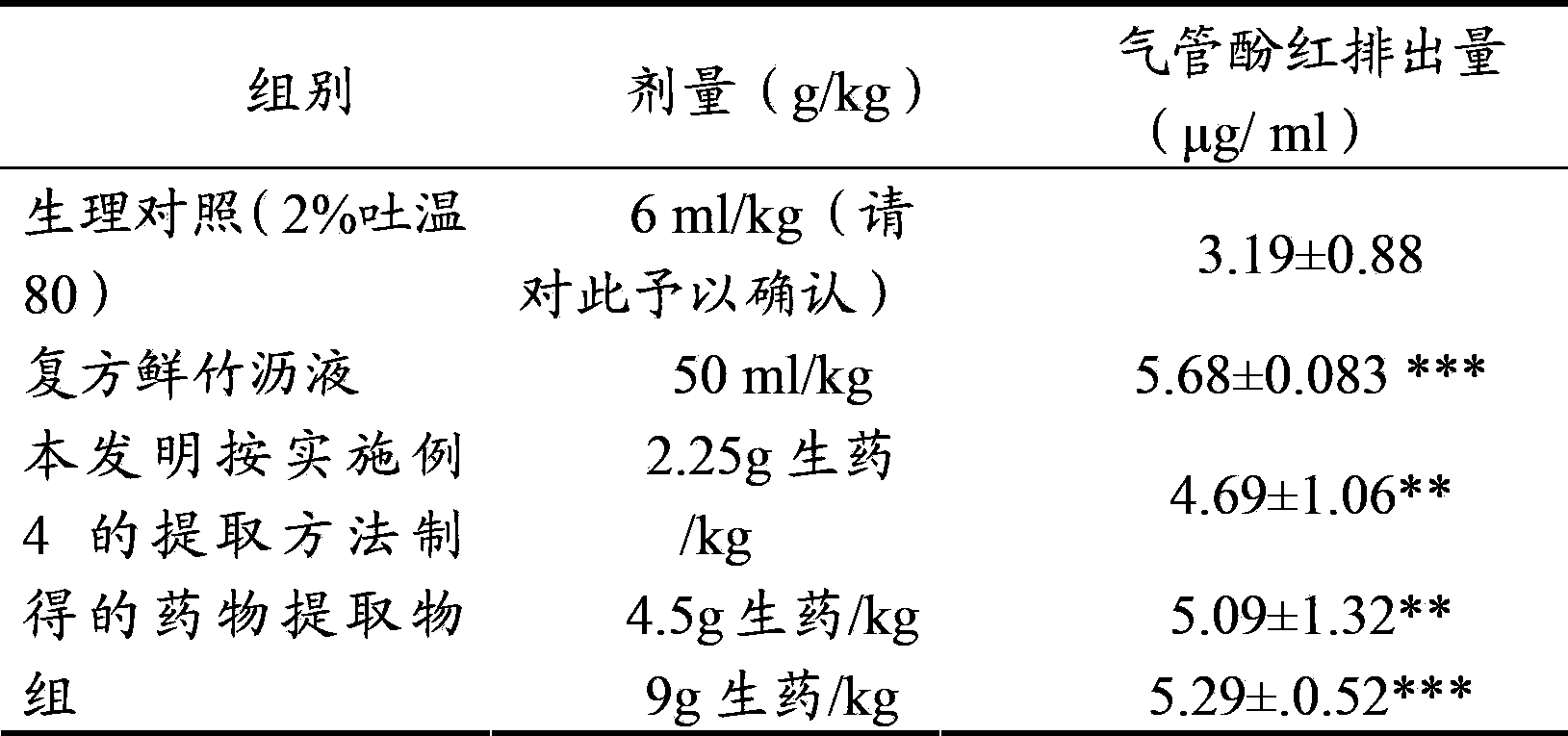
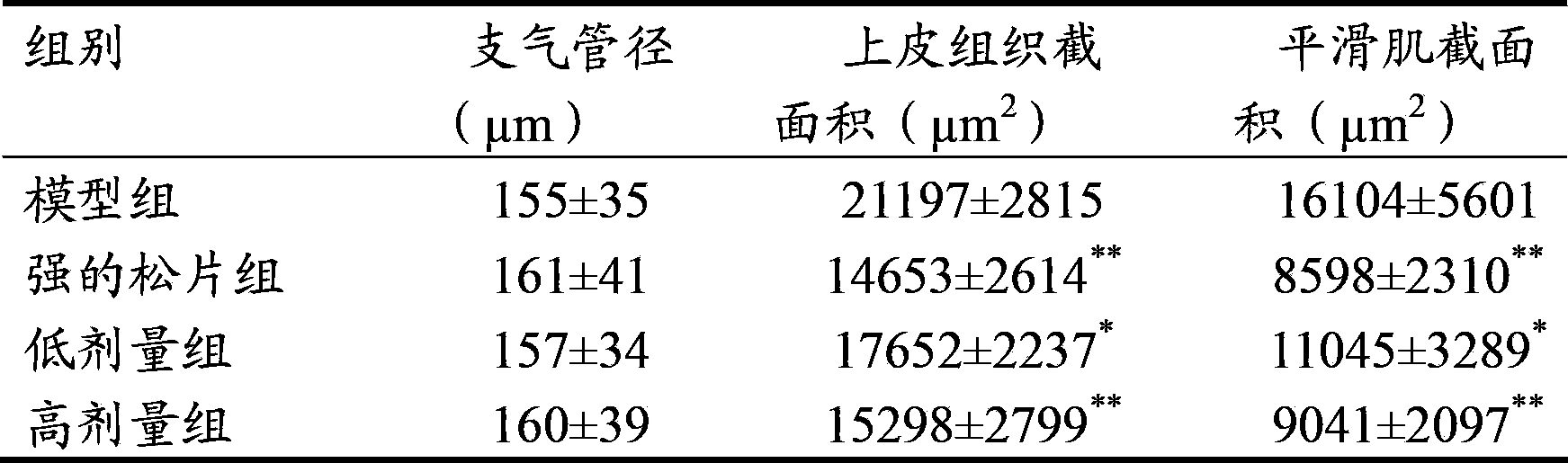
[0037] Get 10kg of aster, 10kg of coltsfoot, add 70% ethanol with 8 times the volume of the total weight of aster and coltsfoot (volume / weight, in L / Kg) to extract twice, each time for 1.5 hours, combine the alcohol extracts, filter, Recover ethanol from the supernatant until it has no alcohol smell, absorb it with 10L wet HPD-100 macroporous resin, and then elute with 30L water and 50L 30% ethanol in sequence, collect the 30% ethanol eluate, recover ethanol, and collect the extract. It was dried under reduced pressure, and the total flavonoid content was 31.4% as rutin, and the total saponin content was 15.7% as aster saponin E.
[0038] Grind the extract obtained above into a fine powder, add an appropriate amount of lactose, wet granulate with about 430ml of 85% ethanol, add 5g of magnesium stearate, 10g of talcum powder, and press into tablets to obtain a system containing 1 part of aster and 1 part of coltsfoot. The resulting pharmaceutical composition in tablets.
Embodiment 2
[0040] Take 10kg of aster and 1kg of coltsfoot, add 80% ethanol with 10 times the volume of the total weight of aster and coltsfoot (volume / weight, in L / Kg) to extract twice, each time for 2 hours, combine the alcohol extracts, filter, Recover ethanol from the supernatant until it has no alcohol smell, absorb it with 10L of wet HPD-300 macroporous resin, and then elute with 40L of water and 40L of 40% ethanol in sequence, collect the 40% ethanol eluate, recover ethanol, and dry under reduced pressure. get the extract. Among them, the total flavonoid content is 42.5% in terms of rutin, and the total saponin content is 17.9% in terms of aster saponin E.
[0041] Grind the extract obtained above into fine powder, mix 500g of microcrystalline cellulose, 300g of hydroxypropyl cellulose, and 40g of micropowdered silica gel, and press into tablets to obtain a dispersible tablet containing 10 parts of aster and 1 part of coltsfoot type of pharmaceutical composition.
Embodiment 3
[0043] Take 1kg of aster and 10kg of coltsfoot, add 12 times the volume of the total weight of aster and coltsfoot (volume / weight, in L / Kg), extract three times with 50% ethanol, each time for 1 hour, combine the alcohol extracts, filter, and put Recover ethanol from the supernatant until it has no alcohol smell, absorb it with 10L wet D101 macroporous resin, elute with 40L water and 40L 60% ethanol in sequence, collect the 40% ethanol eluate, recover ethanol, and dry under reduced pressure to obtain the extract . Among them, the total flavonoid content is 25.8% in terms of rutin, and the total saponin content is 20.9% in terms of aster saponin E.
[0044] Grind the extract obtained above into fine powder, add appropriate amount of PEG4000, melt at 80°C, add the above powder into the melted PEG4000 by solid dispersion technology, and use liquid paraffin at 5°C as coolant to make drop pills. A pharmaceutical composition containing 1 part of aster and 10 parts of coltsfoot made...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com