Synthetic method for phosphate ester
A synthesis method and phosphate ester technology, which are applied in chemical instruments and methods, compounds of Group 5/15 elements of the periodic table, organic chemistry, etc., can solve the problem of large differences in the ratio of mono- and di-esters of phosphate esters and uneven reaction systems , severe local reaction, etc., to achieve good reproducibility, avoid coking and carbonization, and mild reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-11
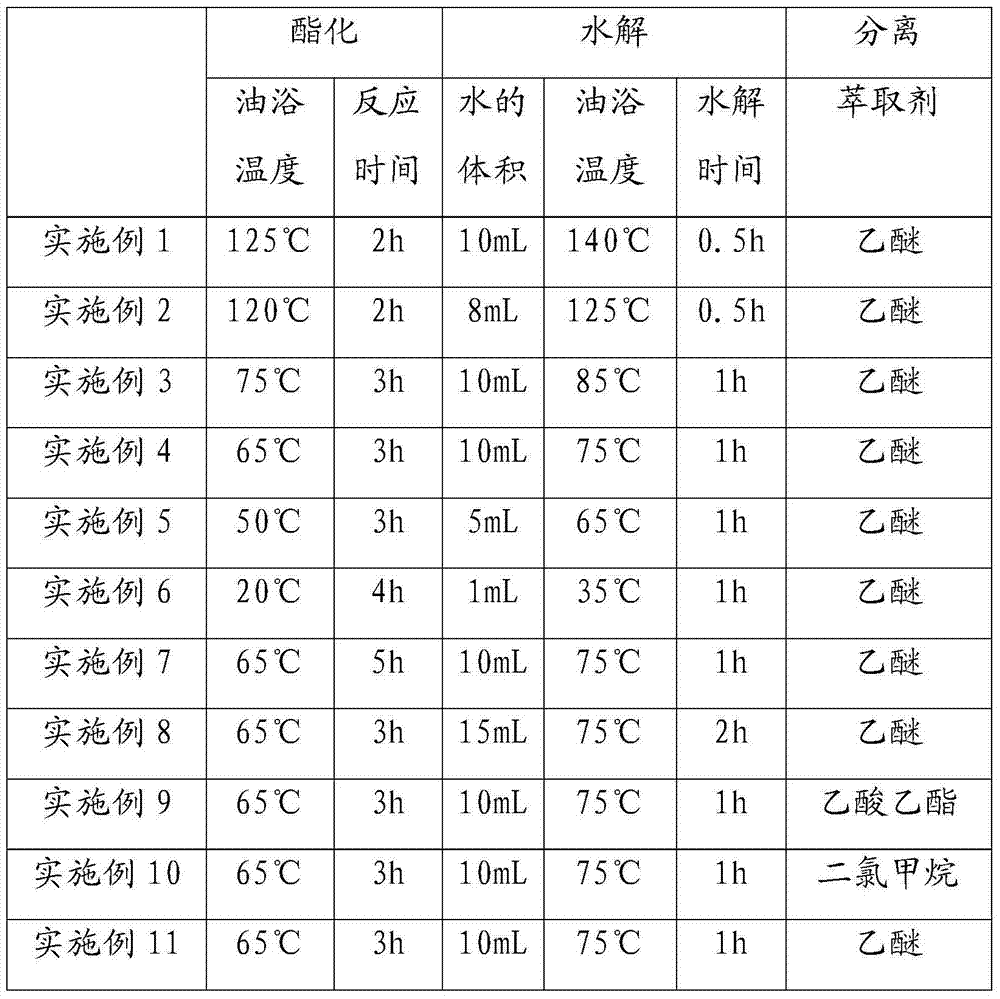
[0018] The reactants and organic solvents in Examples 1-11 are all the same, 0.1 mol of phosphorus pentoxide, 0.2 mol of n-octanol and 100 mL of toluene.
[0019] The preparation method is as follows:
[0020] Mixing: Put phosphorus pentoxide in a beaker, add toluene to the beaker, and stir with a magnetic stirrer to fully disperse phosphorus pentoxide; then divide n-octanol into four parts and add them to the beaker (Example 11, n-octanol was added to the beaker all at once), the interval between the addition of two parts of n-octanol was 10 minutes, and after stirring for 15 minutes, the solution in the beaker changed from milky white to clear.
[0021] Esterification: Transfer the solution in the beaker to a 250mL two-necked bottle and place the two-necked bottle in an oil bath, heat the oil bath to a certain temperature, and maintain this temperature for a period of time.
[0022] Hydrolysis: Add deionized water into the two-necked bottle, and then heat the oil bath to a ...
Embodiment 12-21
[0027] The preparation method of phosphoric acid ester in embodiment 12-21 is as follows:
[0028] Mixing: Put phosphorus pentoxide in a beaker, add 100mL organic solvent into the beaker, stir with a magnetic stirrer to fully disperse phosphorus pentoxide; then divide the primary alcohol into four parts and add them to the beaker one by one, two The interval time of part of primary alcohol adding is 10min, and after continuing to stir for 15min, the solution in the beaker becomes clear by milky white.
[0029] Esterification: Transfer the solution in the beaker to a 250mL two-necked bottle and place the two-necked bottle in an oil bath, heat the oil bath to 65°C (75°C in Example 20-21), and maintain this temperature for 3h ( The reaction time of Examples 19-21 is 3.5h).
[0030] Hydrolysis: add 10 mL of deionized water to the two-necked bottle (the deionized water in Example 19-21 is 5 mL), then heat the oil bath to 75°C (85°C in Example 20-21), and hydrolyze for 1 hour.
[...
Embodiment 22
[0041] Taking Example 4 as a reference, the reaction was amplified 100 times, that is, 10mol, 1.42Kg of phosphorus pentoxide and 20mol, 2.6046kg of n-octanol were reacted.
[0042] Mixing: Put phosphorus pentoxide in a container, add 10L organic solvent into the container, stir with a stirrer to fully disperse phosphorus pentoxide; then divide n-octanol into four parts and add them to the container one by one, The interval between the addition of two parts of n-octanol is 10 minutes, and after stirring for 15 minutes, the solution in the container changes from milky white to clear.
[0043] Esterification: Transfer the solution in the container to a double-ported reaction tank and heat the reaction tank to 65°C, maintaining this temperature for 3 hours.
[0044] Hydrolysis: Add 1L of deionized water to the reaction tank, then continue heating to raise the temperature of the reaction tank to 75°C, and hydrolyze for 1 hour.
[0045] Separation: Stop heating the reaction tank af...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com