Energetic material 4, 4, 8, 8-tetranitroadamantane-2, 6-dinitrate and preparation method thereof
A technology of tetranitroadamantane and dinitrate is applied in the preparation of nitro compounds, nitrated acyclic/alicyclic/heterocyclic amine explosive compositions, organic chemistry and other directions, and can solve the problems of many steps, high cost and high yield. Low problems, to achieve the effect of simple processing, easy product and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0039] Preparation of 1,5-cyclooctanediol
[0040]
Embodiment 1
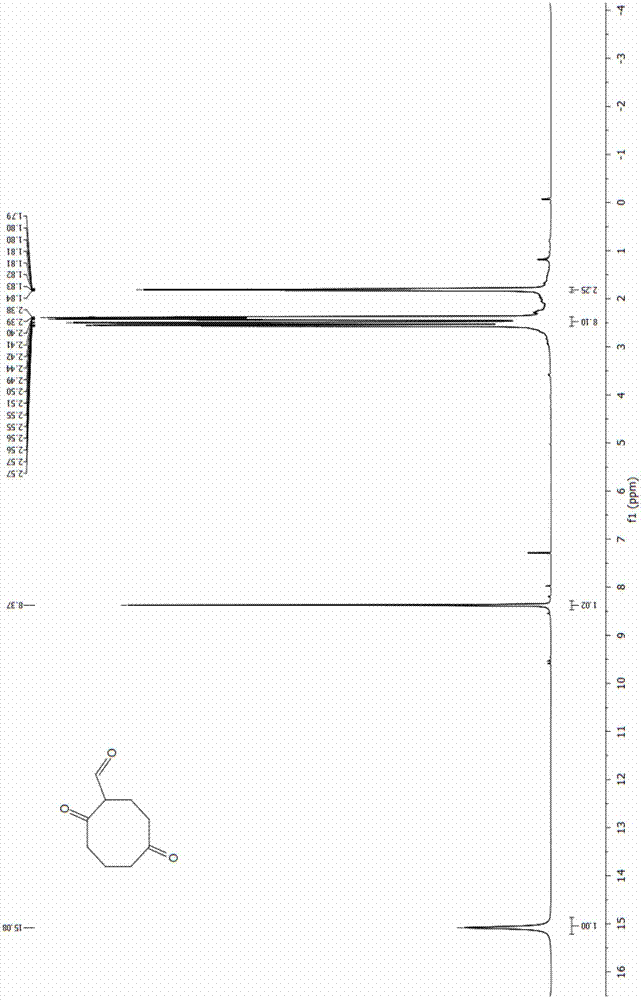
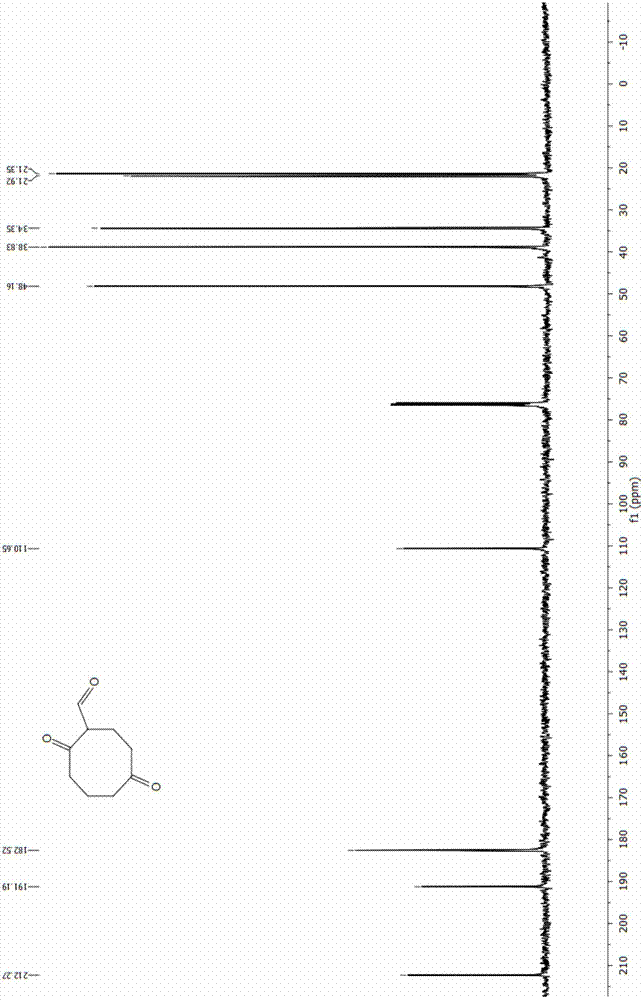
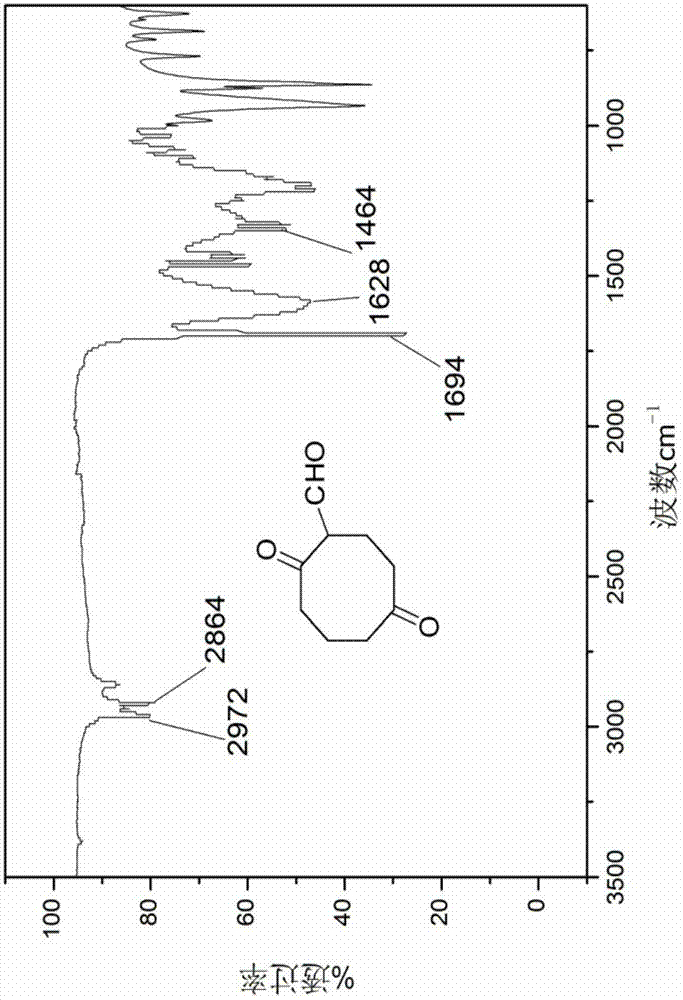
[0042] N 2 Under protective conditions, 20 mL of 1M borane tetrahydrofuran complex (20 mmol) was added into a three-necked flask. Add 2.45 mL of 1,5-cyclooctadiene (20 mmol) into a dropping funnel with 2.55 mL of tetrahydrofuran, and slowly drop it into a three-neck flask under ice-cooling conditions. After the dropwise addition was completed, the reaction was carried out at room temperature for 1 h. Then 8 mL of 3 M NaOH aqueous solution was slowly added dropwise to the system. After the NaOH aqueous solution was added dropwise, 12 mL of 30% hydrogen peroxide solution was added dropwise to the system, and reacted at 40 °C for 4 h after the dropwise addition was completed. After the reaction was completed, an appropriate amount of K was added 2 CO 3 The solution was separated, extracted 3-6 times with ethyl acetate, and the organic phase was washed with anhydrous Na 2 SO 4 After drying, the solvent was distilled off under reduced pressure to obtain 2.6 g of a colorless v...
Embodiment 2
[0046] Add 55 mL of dichloromethane and 4.13 mL of oxalyl chloride (48.05 mmol) into the three-necked flask successively, and cool to -68 °C. N 2 Under protection, 6.62 mL DMSO (93.2 mmol) was slowly added dropwise to the system, and stirring was continued for 15 min after the dropwise addition was complete. 3 g of 1,5-cyclooctanediol (20.8 mmol) was dissolved in 15 mL of DMSO, and slowly added dropwise to the flask, the solution became cloudy, and the reaction was continued at low temperature for 70 min after the addition was completed. Continue to add 29.7 mL of triethylamine (213 mmol) dropwise at low temperature, continue to stir for 30 min, and let the reaction system slowly rise to room temperature within 1 h. After the reaction, the reaction system was washed with 150 mL of a saturated aqueous solution of ammonium chloride and sodium bicarbonate, extracted with dichloromethane, the organic phase was dried over anhydrous sodium sulfate, and the solvent was distilled off...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com