Citral submicron emulsion and preparation method thereof
A citral and sub-microemulsion technology, which is applied in pharmaceutical formulations, emulsion delivery, aldehyde active ingredients, etc., can solve problems such as undiscovered citral formulations, few formulations and unstable properties of citral oil. Not easy to change compatibility, promote drug absorption, good compatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Composition of citral submicron emulsion:
[0024] Citral: emulsifier: glycerin: ultrapure water = 1:0.5:0.25:2 (volume ratio), the emulsifier is a mixture of Tween 80: Span 80 = 1:2 (volume ratio), and the amount of lecithin is The volumetric dosage of citral is 0.005g / ml.
[0025] Preparation method: at 25°C, take 0.33ml Tween 80, 0.66ml Span 80, 0.5ml glycerin, and 10mg lecithin, stir and mix at a speed of 10000r / min, add 2ml citral to form an oil phase, Add 4ml of ultrapure water to the above oil phase, and stir it for 4min at a speed of 15500r / min in an emulsifier (model IKA T18, manufacturer: IKA Group Co., Ltd., Germany) to obtain oil-in-water (O / W) type citral submicron emulsion.
[0026] Performance test of citral submicron emulsion:
[0027] (1) Determination of encapsulation efficiency of citral submicron emulsion:
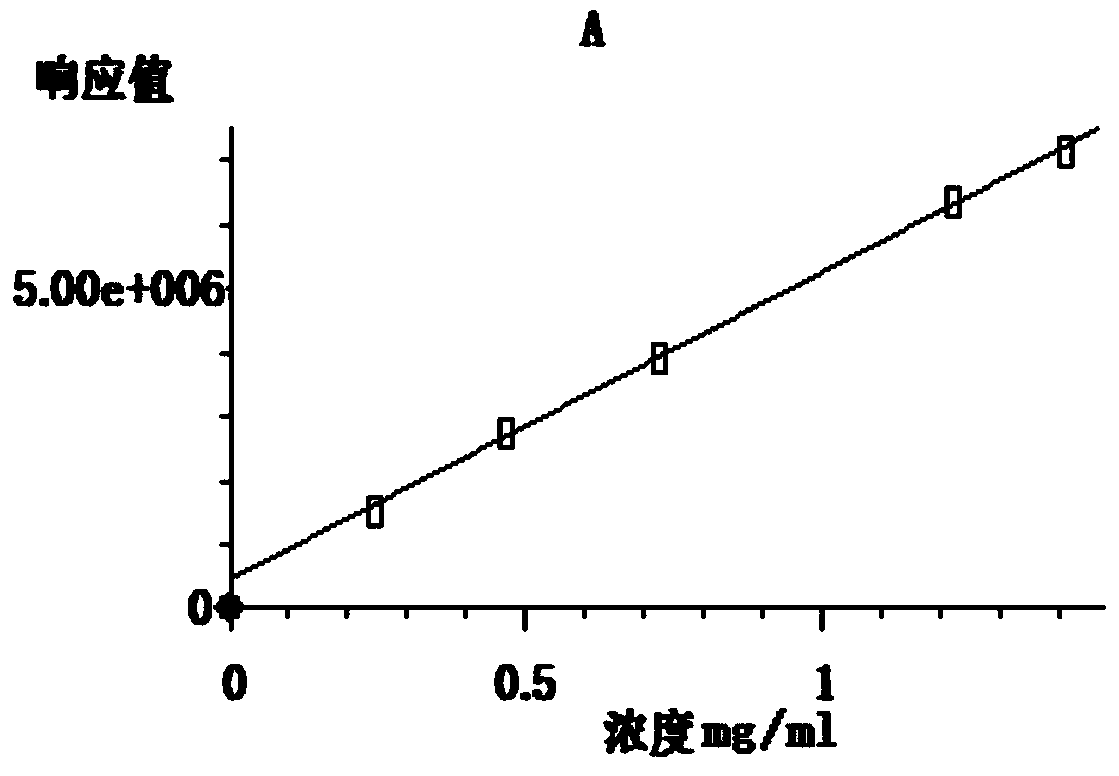
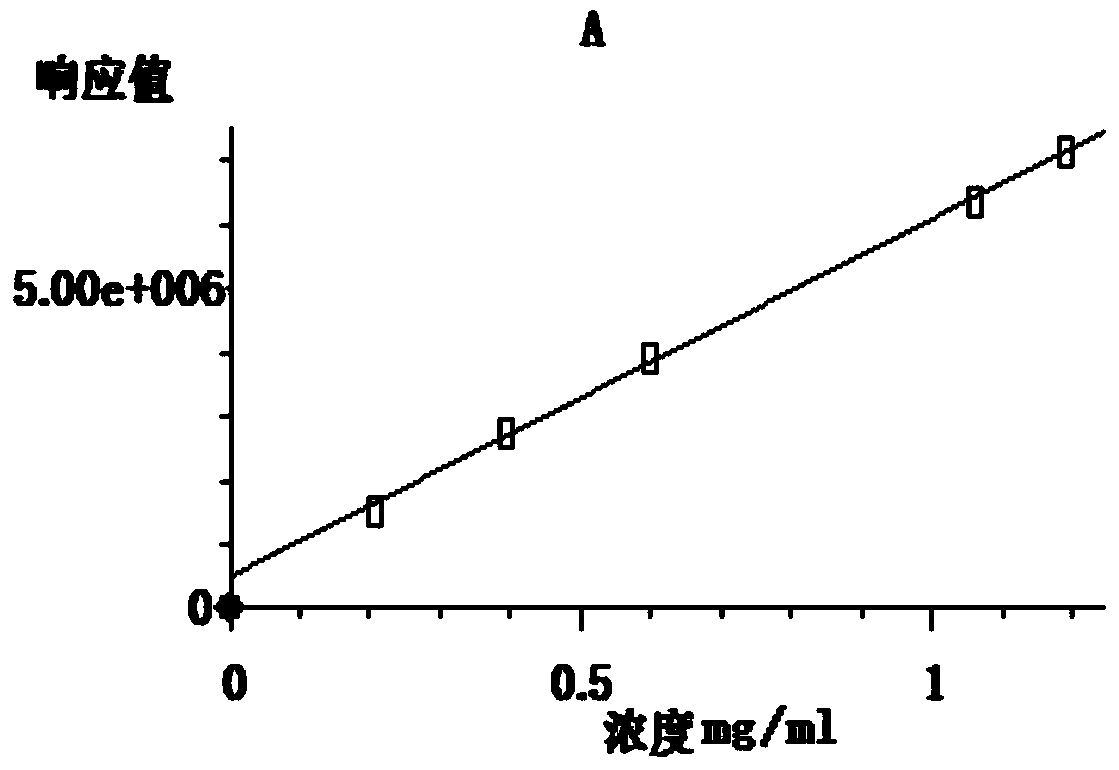
[0028] Mainly use GC-MS (model 7890A GC-5975C MSD, manufacturer: Agilent, USA), conditions: chromatographic column HP-5MS5%Phenyl Methyl Si...
Embodiment 2
[0043] Composition of citral submicron emulsion:
[0044] Citral: emulsifier: polyethylene glycol: ultrapure water = 1:1:0.25:2 (volume ratio), the emulsifier is a mixture of Tween 80: Span 80 = 1:3 (volume ratio), lecithin The mass dosage is 0.01g / ml based on the volumetric dosage of citral.
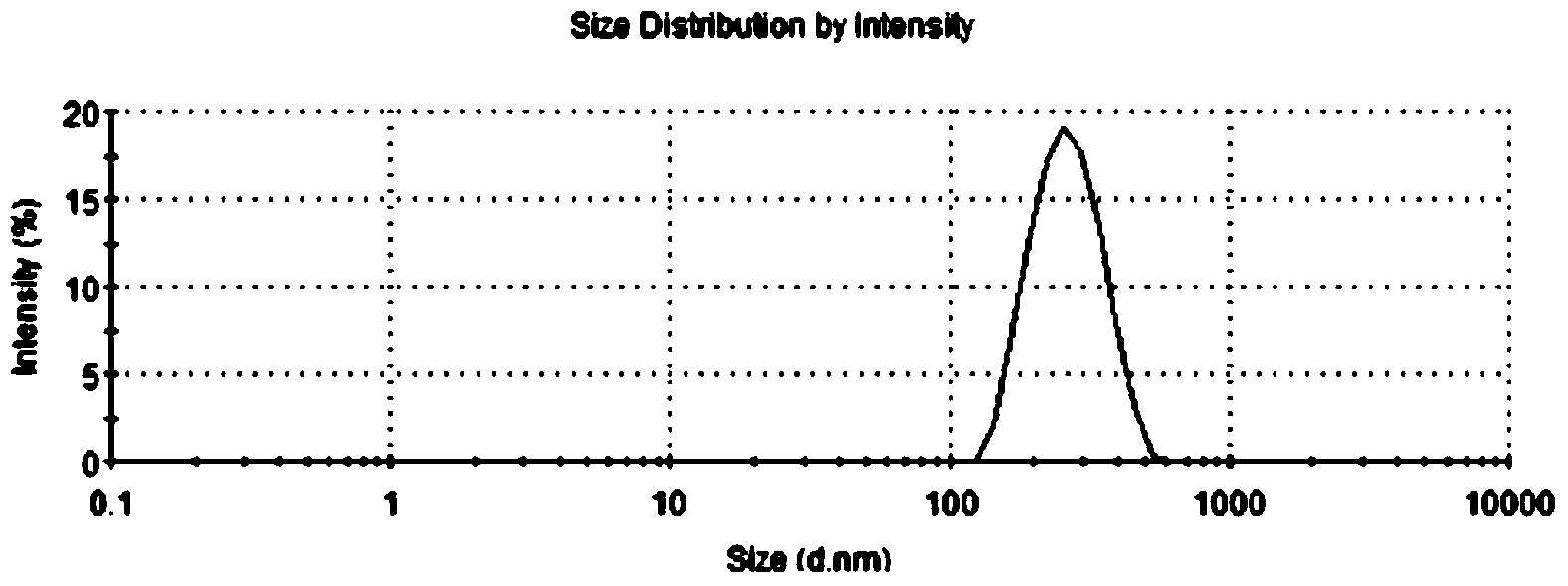
[0045] The preparation method is the same as in Example 1, and the temperature condition is 20°C. The performance test method of embodiment 2 citral submicron emulsion is the same as embodiment 1. The average encapsulation efficiency of three batches of citral submicron emulsions was measured to be 87%, the polydispersity index PDI was 0.204, the average particle size was 269nm, the average Zeta potential was -27.6mV, and the stability constant K E The value is 0.20. The results of the stability test showed that the three batches of citral submicroemulsion had good stability.
Embodiment 3
[0047] Composition of citral submicron emulsion:
[0048] Citral: emulsifier: glycerin: ultrapure water=1:2:0.25:2 (volume ratio), the emulsifier is a mixture of Tween 80: Span 80=1:1 (volume ratio), and the amount of lecithin is The volumetric dosage of citral is 0.005g / ml.
[0049] The preparation method is the same as in Example 1, and the temperature condition is 10°C. Embodiment 3 The performance testing method of the citral submicron emulsion is the same as that in Embodiment 1. The average encapsulation efficiency of three batches of citral submicron emulsions was measured to be 83%, the polydispersity index PDI was 0.219, the average particle size was 260nm, the average Zeta potential was -26.5mV, and the stability constant K E The value is 0.29. The results of the stability test showed that the three batches of citral submicroemulsion had better stability.
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrical resistivity | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| electric potential / voltage | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com