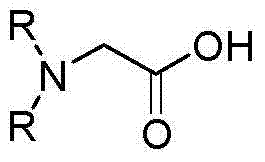
Clean and environment-friendly production method of N,N-dialkylglycine
A technology of dialkylglycine and barium dialkylglycine, which is applied in the field of preparation of N,N-dialkylglycine, can solve the problems of difficult biochemical treatment of wastewater, difficulty in separation and purification, and low product yield, and achieve purity And the effect of high yield, easy separation and purification, high yield and purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
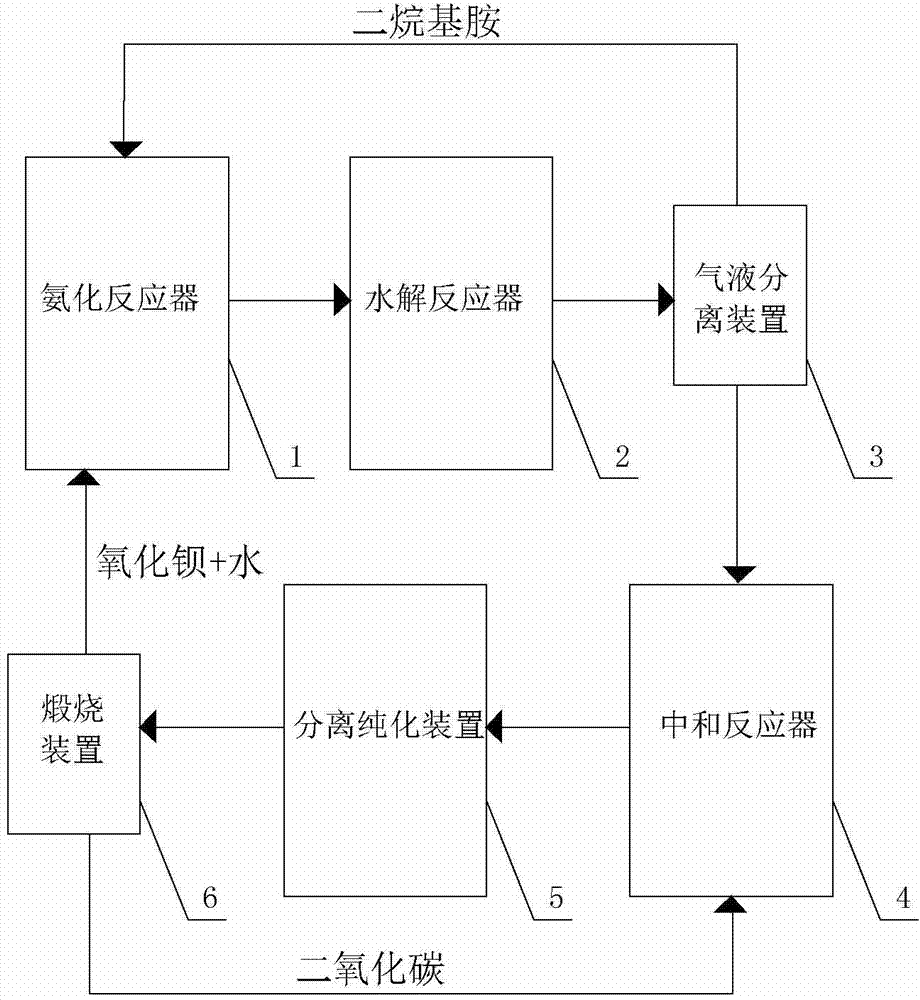
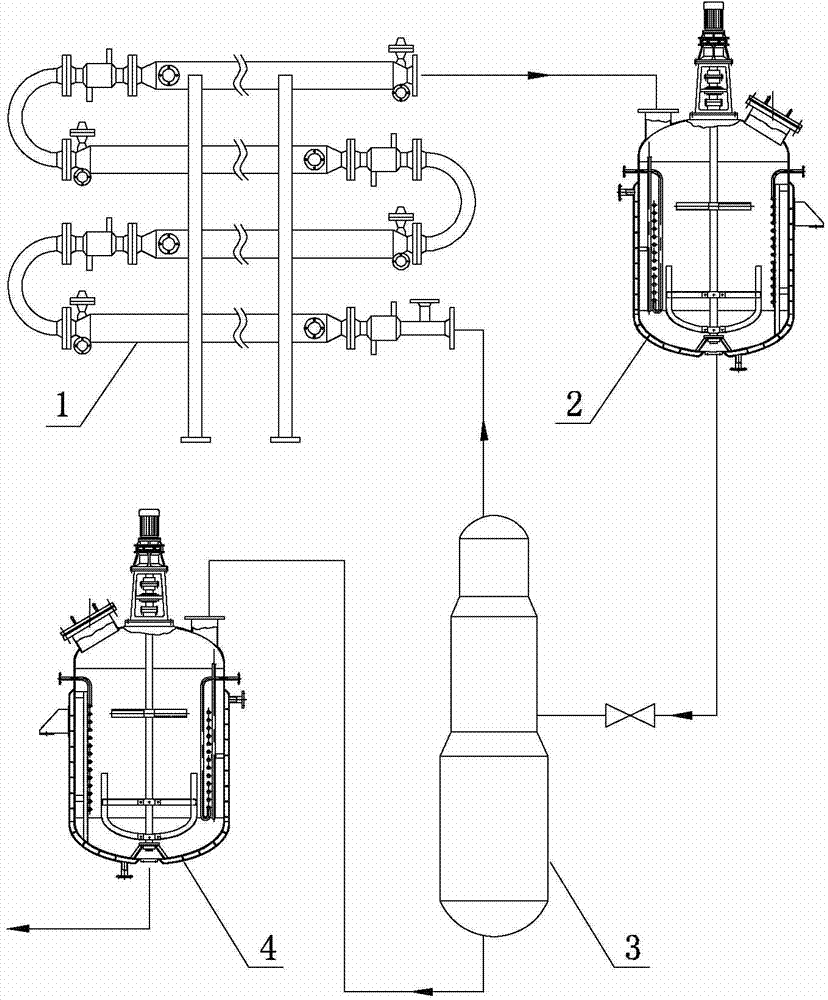
[0061] At room temperature, mix 1000g of 40% hydroxyacetonitrile aqueous solution with 1435g of 33% dimethylamine aqueous solution, then immediately use the feed pump to pump the resulting mixture into the tubular reactor, then raise the temperature to 80°C, and the gauge pressure is 1.0MPa , the residence time of the reaction material in the tubular reactor is 15 minutes, and the liquid flowing out from the outlet of the tubular reactor is directly added to a four-necked round bottom flask with 1106g60% barium hydroxide 3000ml, and the hydrolysis reaction temperature The temperature is 85°C, and the reaction time is 3 hours. After the hydrolysis reaction is completed, the hydrolyzed liquid is transferred to the gas stripping equipment for gas-liquid separation, and the dimethylamine is recovered. The liquid after the gas stripping is transferred to the high-pressure reactor and heated to 80 ℃, then carbon dioxide is introduced, the pressure of carbon dioxide is 0.2MPa, and the...
Embodiment 2
[0065] At room temperature, mix 1000g of 40% hydroxyacetonitrile aqueous solution with 1435g of 33% dimethylamine aqueous solution, then immediately use the feed pump to pump the resulting mixture into the tubular reactor, then raise the temperature to 80°C, and the gauge pressure is 1.0MPa , the residence time of the reaction material in the tubular reactor was 15 minutes, and the liquid flowing out from the discharge port of the tubular reactor was directly added to the solution containing 1106g60% barium hydroxide (the barium hydroxide was reclaimed in Example 1 and obtained). In a 3000ml four-necked round bottom flask, the hydrolysis reaction temperature is 85°C, and the reaction time is 3 hours. After the hydrolysis reaction is completed, the hydrolyzate is transferred to an air stripping device for gas-liquid separation, and dimethylamine is recovered. , the liquid after air stripping is transferred to a high-pressure reactor, heated to 80°C, and then carbon dioxide is in...
Embodiment 3
[0069] At room temperature, mix 1000g of 40% hydroxyacetonitrile aqueous solution with 1435g of 33% dimethylamine aqueous solution, then immediately use the feed pump to pump the resulting mixture into the tubular reactor, then raise the temperature to 80°C, and the gauge pressure is 1.0MPa , the residence time of the reaction material in the tubular reactor was 15 minutes, and the liquid flowing out from the discharge port of the tubular reactor was directly added to the 1325.6g50% barium hydroxide (this barium hydroxide was reclaimed in Example 2) obtained barium hydroxide) in a 3000ml four-necked round-bottomed flask, the hydrolysis reaction temperature was 85°C, and the reaction time was 3 hours. After the hydrolysis reaction was completed, the hydrolyzed solution was transferred to an air stripping device for gas-liquid separation, and dimethylformazol was recovered. Amine, the liquid after air stripping is transferred to a high-pressure reactor, heated to 80°C, and then c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com