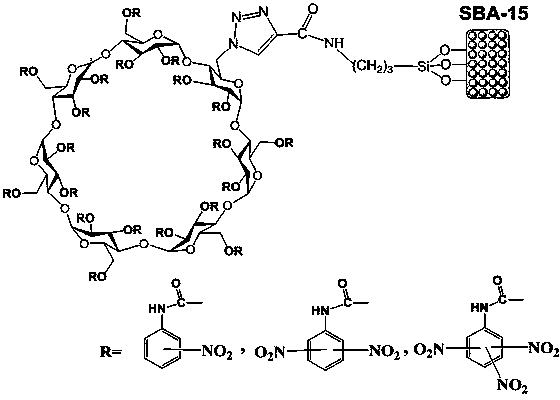
Preparation method of nitrophenylcarbamate full-derived beta-cyclodextrin bonded ordered mesoporous SBA-15 chiral stationary phase
A nitrophenylcarbamate, chiral stationary phase technology, applied in chemical instruments and methods, other chemical processes and other directions, can solve the problems of high mobile phase cost and stationary phase loss, and achieves low preparation cost and stable performance. , the effect of high bonding
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Take SBA-15 (400 m 2 / g) Activated silica gel 2.5 g as the base.
[0025] (1) Under a nitrogen atmosphere, according to the ratio of β-cyclodextrin (mmol): anhydrous pyridine (mL): p-toluenesulfonyl chloride (mmol): anhydrous pyridine (mL) of 1 : 10 : 0.9 : 2.5, Dissolve β-cyclodextrin in anhydrous pyridine, dissolve p-toluenesulfonyl chloride in anhydrous pyridine and add dropwise to the above solution, stir for 2 h, and stir at room temperature for 10 h. After the solution turns yellow, remove pyridine in vacuum, add Anhydrous diethyl ether gave a white solid, which was filtered and recrystallized several times with ultrapure water to obtain the 6-oxo-tosylated-β-cyclodextrin intermediate ( ). Under nitrogen atmosphere, according to the intermediate ( ) (mmol): sodium azide (mmol): anhydrous N, N-dimethylformamide (mL) is a ratio of 1: 0.9: 12, the intermediate ( ) was dissolved in anhydrous N,N-dimethylformamide, added sodium azide, and stirred at room tempera...
Embodiment 2
[0033] Take SBA-15 (500 m 2 / g) Activated silica gel 2.5 g as the base.
[0034] (1) Under nitrogen atmosphere, according to the ratio of β-cyclodextrin (mmol): anhydrous pyridine (mL): p-toluenesulfonyl chloride (mmol): anhydrous pyridine (mL) of 1 : 12 : 1.1 : 3.0, Dissolve β-cyclodextrin in anhydrous pyridine, dissolve p-toluenesulfonyl chloride in anhydrous pyridine, add dropwise to the above solution and stir for 3 h, and stir at room temperature for 12 h. After the solution turns yellow, remove pyridine in vacuum, add Anhydrous diethyl ether gave a white solid, which was filtered and recrystallized several times with ultrapure water to obtain the 6-oxo-tosylated-β-cyclodextrin intermediate ( ). Under nitrogen atmosphere, according to the intermediate ( ) (mmol): sodium azide (mmol): anhydrous N, N-dimethylformamide (mL) is a ratio of 1: 1.1: 14, the intermediate ( ) was dissolved in anhydrous N,N-dimethylformamide, added sodium azide, and stirred at room temperatu...
Embodiment 3
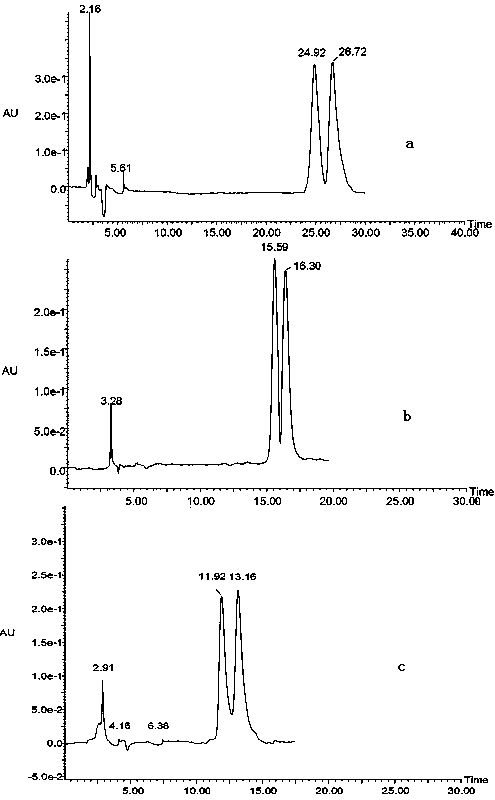
[0041] Embodiment 3 split experiment
[0042] The fully derivatized β-cyclodextrin-bonded SBA-15 silica gel chiral stationary phase containing nitrophenylcarbamate in Example 3 was used for the separation of chiral drugs.
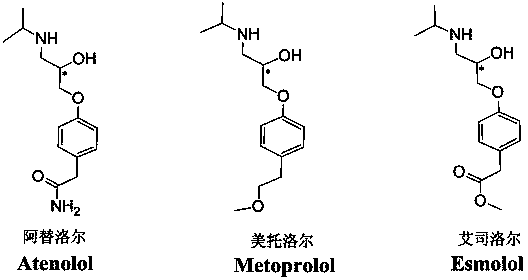
[0043] Split object: three β-receptor blockers atenolol, metoprolol, esmolol structure see figure 2 .
[0044]Specific process: The chromatographic column is filled by the homogenate filling method. Using acetone as the homogenizer and methanol as the displacing agent, the homogenate was packed into a cleaned chromatographic column (150 mm × 4.6 mm I.D.) under a pressure of 5000 psi, and the pressure was maintained for 30 min. Remove the packed chromatographic column, put on the chromatographic column head, and mark the direction of mobile phase. Rinse the chromatographic column repeatedly with water and methanol, equilibrate with the mobile phase used, and inject the sample after the baseline is stable. The standard substance was made into a stock sol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com