Polymer containing thiophene sulfone and preparation method thereof, and organic electroluminescent device
A polymer, thiophene sulfone technology, applied in the field of organic electroluminescent devices, can solve problems such as poor thermal stability, poor luminous efficiency, and short component life, achieve novel structure, solve low efficiency problems, and film formation. Excellent performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
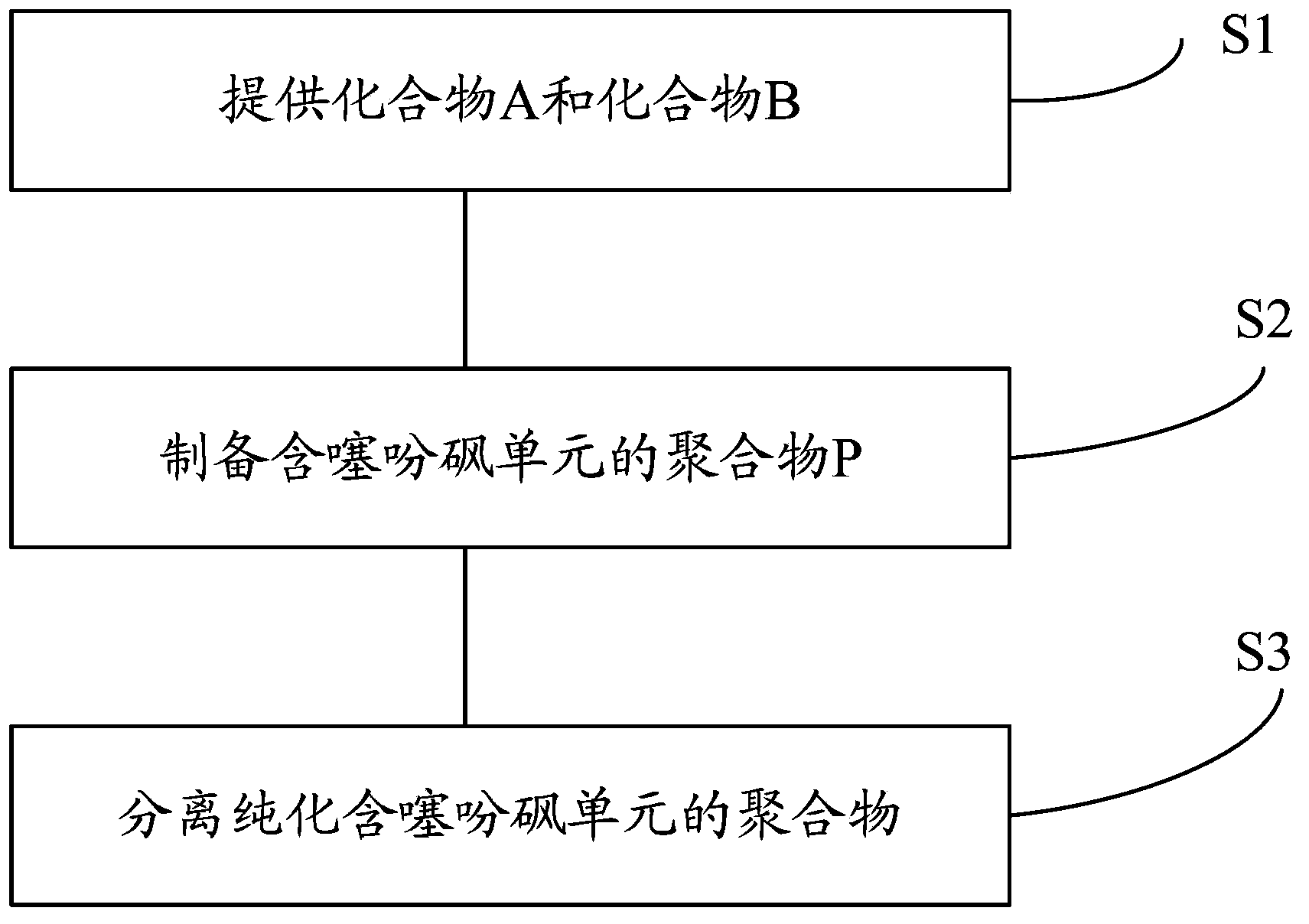
[0037] The preparation method of the polymer containing thiophene sulfone of one embodiment, such as figure 1 shown, including the following steps:
[0038] Step S1, providing compound A and compound B.
[0039] The structural formula of compound A is: Among them, R 1 for C 1 ~C 20 of alkyl.
[0040] The structural formula of compound B is:
[0041] Step S2, preparing polymer P containing thiophene sulfone.
[0042] In an oxygen-free environment, add compound A and compound B at a molar ratio of 1:1 to 1:1.2 into an organic solvent containing a catalyst and an alkali solution, and perform a Suzuki coupling reaction at 70°C to 130°C for 12 hours to 96 hours , the catalyzer is the mixture of organic palladium or organic palladium and organic phosphorus ligand, obtains the polymer P containing thiophene sulfone represented by the following structural formula:
[0043]
[0044] Wherein, n is an integer of 10-100.
[0045] In this embodiment, the Suzuki coupling reac...
Embodiment 1
[0064] This example discloses poly{9,9-di-n-octylfluorene-2,7-diyl-co-thiophene sulfone-2,5-diyl} (polymer P1 containing thiophene sulfone) with the following structural formula:
[0065]
[0066] The preparation process of the above-mentioned polymer P1 containing thiophene sulfone is as follows:
[0067] Under argon protection, 9,9-di-n-octylfluorene-2,7-dipinacol borate (128 mg, 0.2 mmol), 2,5-dibromothiophene sulfone (54 mg, 0.2 mmol) were added In the flask filled with 10ml of toluene solvent, after fully dissolving, potassium carbonate (2mL, 2mol / L) solution was added into the flask, vacuumized to deoxygenate and filled with argon, then added bistriphenylphosphine palladium dichloride ( 5.6mg, 0.008mmol); the flask was heated to 100°C for Suzuki coupling reaction for 48h. Subsequently, the polymerization reaction was stopped after cooling down, and 50 ml of methanol was added dropwise to the flask for sedimentation; after filtering through a Soxhlet extractor, the mi...
Embodiment 2
[0075] This example discloses poly{9,9-dimethylfluorene-2,7-diyl-co-thiophene sulfone-2,5-diyl} (polymer P2 containing thiophene sulfone) with the following structural formula:
[0076]
[0077] The preparation process of the above-mentioned polymer P2 containing thiophene sulfone is as follows:
[0078] Under the protection of mixed gas of nitrogen and argon, 9,9-dimethylfluorene-2,7-dipinacol borate (89mg, 0.3mmol), 2,5-dibromothiophene sulfone (82mg, 0.3mmol ) and 15mL tetrahydrofuran into a 50mL two-necked bottle, fully dissolved, and then a mixed gas of nitrogen and argon was introduced to exhaust the air for about 20 minutes, then tetrakistriphenylphosphine palladium (4mg, 0.003mmol) was added into it, and after fully dissolving Then add sodium bicarbonate (3mL, 2mol / L) solution, and then fully ventilate the mixed gas of nitrogen and argon to exhaust the air for about 10min, then heat the two-necked flask to 70°C for Suzuki coupling reaction for 60h. Subsequently, th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com