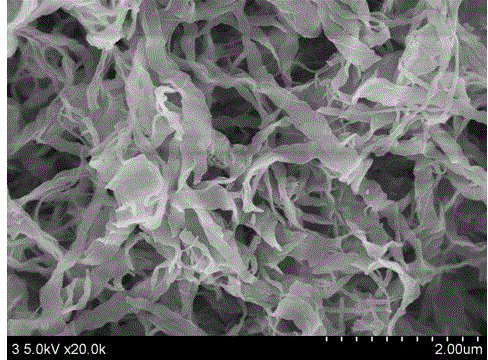
Carbon nanoribbon with large specific surface area and preparation method thereof
A nanobelt and porous carbon technology, applied in the fields of nanotechnology, nanotechnology and nanotechnology for materials and surface science, can solve the problems of complex process, limited application prospects of porous carbon, high cost, etc. The effect of easy industrial production and short process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] 1. Preparation of nanobelts
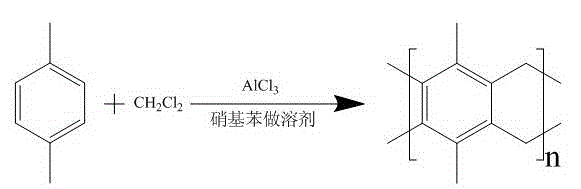
[0035] Dissolve 1.06 grams of p-xylene in 100 mL of nitrobenzene, add 13.4 grams of anhydrous aluminum trichloride, stir evenly, add 2.0 grams of dichloromethane, stir and react for 2 hours, and then transfer to a polytetrafluoroethylene reactor. React at 100°C for 12 hours, cool naturally, filter, wash with ethanol, and dry to obtain a brown-black spongy powder.
[0036] The chemical equation for this reaction is as follows:
[0037]
[0038] 2. Carbonization
[0039] Weigh 1.0 g of the above powder, put it into a crucible, raise the temperature from 5°C to 500°C per minute under an inert atmosphere, and keep it warm for 10 hours to obtain 0.76 g of black powder.
[0040] 3. Activation
[0041] Take 0.76 g of carbonized powder, add 0.76 g of KOH, grind evenly, transfer to an atmosphere furnace, raise the temperature to 700°C at 5°C per minute under an inert atmosphere, keep it warm for 4 hours, soak the obtained powder in water for 2...
Embodiment 2
[0045] 1. Preparation of nanobelts
[0046] Dissolve 1.34 grams of p-diethylbenzene in 100 mL of nitrobenzene, add 6.4 grams of boron trifluoride, stir evenly, add 2.0 grams of dichloromethane, stir and react for 2 hours, then transfer to a polytetrafluoroethylene reactor, React at 100°C for 12 hours, filter after natural cooling, wash with ethanol, and dry to obtain 1.5 g of brown-black spongy powder.
[0047] 2. Carbonization
[0048] Weigh 1.0 g of the above powder, put it into a crucible, raise the temperature from 5 °C to 800 °C per minute under an inert atmosphere, and keep it warm for 2 hours to obtain 0.69 g of black powder.
[0049] 3. Activation
[0050] Take 0.69 grams of carbonized powder, add 6.9 grams of K 2 CO 3 , grind evenly, transfer to an atmosphere furnace, heat up to 850°C at 5°C per minute under an inert atmosphere, keep warm for 4 hours, soak the obtained powder in water for 2 hours, filter, wash with dilute hydrochloric acid, wash with water, and dr...
Embodiment 3
[0054] 1. Preparation of nanobelts
[0055] Dissolve 1.10 grams of hydroquinone in 100 mL of water, add 10 grams of concentrated sulfuric acid, stir well, add 2.0 grams of dichloromethane, stir well, then transfer to a polytetrafluoroethylene reactor, react at 190 ° C for 12 hours, after natural cooling Filter, wash with ethanol, and dry to obtain 1.3 g of brown-black spongy powder.
[0056] 2. Carbonization
[0057] Weigh 1.0 g of the above powder, put it into a crucible, raise the temperature to 900 °C at 5 °C per minute under an inert atmosphere, and keep it warm for 2 hours to obtain 0.64 g of black powder.
[0058] 3. Activation
[0059] Take 0.6 grams of carbonized powder, add 3.6 grams of ZnCl 2 , grind evenly, transfer to an atmosphere furnace, heat up to 650°C per minute at 5°C under an inert atmosphere, keep warm for 6 hours, soak the obtained powder in dilute hydrochloric acid for 6 hours, wash with dilute hydrochloric acid, wash with water, and dry to obtain 0.3...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com