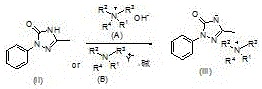A kind of method for synthesizing sulfentrazone intermediate and sulfentrazone
A technology of sulfentrazone and intermediates, applied in the field of synthesis of organic fluorine compounds, can solve problems affecting product quality or yield, generation of formamide impurities, poor atom economy, etc., to achieve high product quality, less side reactions, The effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
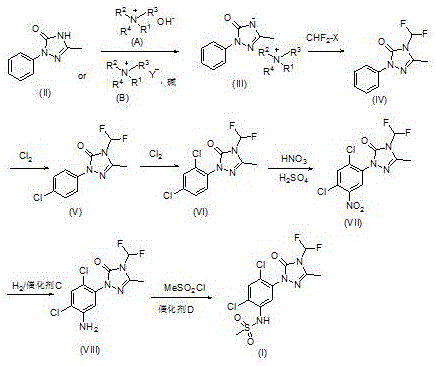
[0034] The preparation method of compound (III) comprises: using compound (II) as a raw material, reacting in an aprotic solvent under the action of compound (A) or compound (B) and a base,
[0035]
[0036] Wherein R1, R2, R3, R4 are C1-C18 alkyl or C6-C10 aryl, and R1, R2, R3, R4 are the same or different.
[0037] R1, R2, R3, R4 of the compound (A) are preferably all n-butyl groups; the molar ratio of the compound (A) to the compound (II) is preferably 0.1-1.2:1; the reaction temperature is preferably 50 ~150°C. R1, R2, R3 and R4 of the compound (B) are preferably all n-butyl, Y is preferably bromine; the molar ratio of compound B to compound (II) is preferably 0.1-1.2:1. Described alkali is alkali metal hydroxide or carbonate, and described alkali metal hydroxide is preferably potassium hydroxide, and described carbonate is preferably potassium carbonate; The mole of potassium hydroxide and compound (II) The ratio is preferably 1-1.5:1, and the molar ratio of potassiu...
Embodiment 1
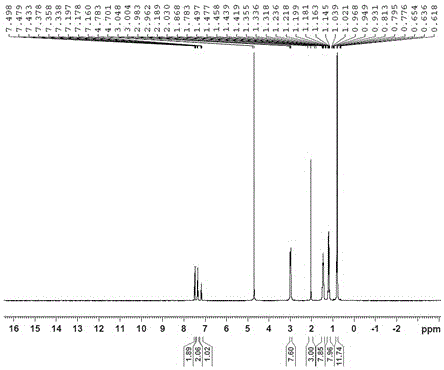
[0040] Put 1-phenyl-3-methyl-1H-1,2,4-triazol-5-one (2g), toluene (40mL), and 11.6g of tetrabutylammonium hydroxide aqueous solution (content 25%) into a 100mL flask . The reaction mixture was stirred and heated to 110°C for reaction, refluxed with water, and dried under vacuum to obtain compound (III, 1-phenyl-3-methyl-1,2,4-triazol-5-one tetrabutylammonium) (4.7 g). 1HNMR (400MHz, D2O) δ7.49 (2H, d, J = 7.6 Hz), 7.36 (2H, dd, J = 7.6, 7.6 Hz), 7.18 (1H, d, J = 7.6 Hz), 2.98 (8H, t, J = 8.4 Hz), 2.03 (3H, s), 1.46 (8H, m), 1.19 (8H, m), 1.19 (8H, m), 0.80 (12H, t, J = 8.4 Hz).
Embodiment 2
[0042] Put 1-phenyl-3-methyl-1H-1,2,4-triazol-5-one (50g), toluene (300mL), and 296g of tetrabutylammonium hydroxide aqueous solution (content 25%) into a 1000mL flask. Stir the reaction mixture to heat up and react, and reflux with water, pass through difluorochloromethane at 80-90°C until the reaction is complete, remove the solvent, add water (200mL), stir, filter, and dry to obtain 1-phenyl-4-difluoromethane Ethyl-3-methyl-1H-1,2,4-triazol-5-one (60 g).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com