Rabeprazole correlate D synthesis method
A technology of rabeprazole and a synthetic method, which is applied in the field of medicine, can solve problems such as many side reactions, low yield, and difficult control of oxidation reaction, and achieve the effect of increasing yield and increasing yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
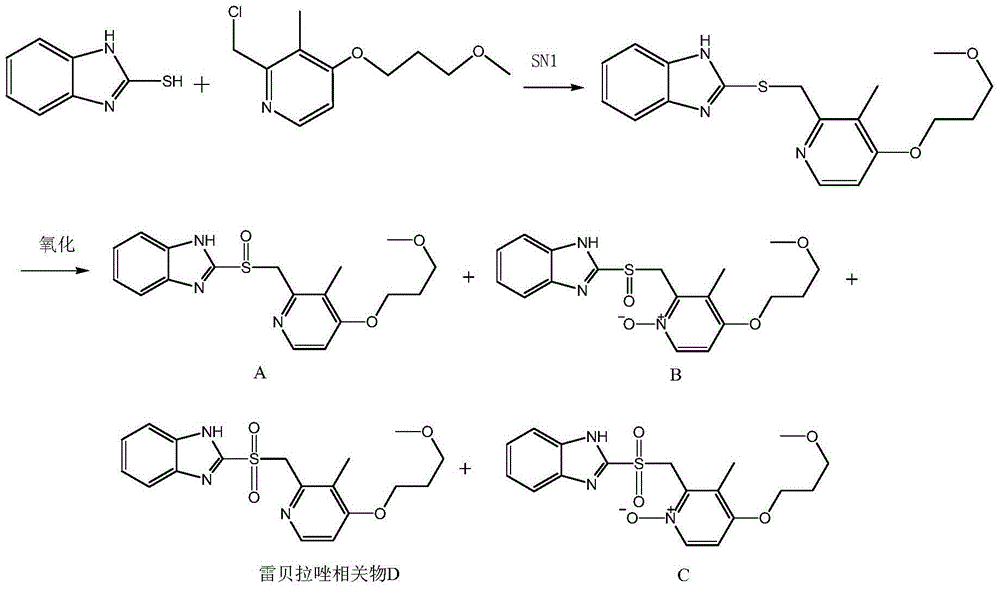
[0034] 1.1 Synthesis of thioether compound——2-[[[4-(3-methoxypropoxy)-3-methyl-2-pyridyl]methyl]thio]benzimidazole
[0035] At room temperature, add 250 mL of analytically pure acetonitrile, 15.0 g (0.1 mol) of 2-mercaptobenzimidazole, and 13.3 g (0.05 mol) of 2-chloromethyl-3-methyl-4- (3-methoxypropoxy)pyridine hydrochloride and 13mL of triethylamine were stirred at room temperature for 24h. The reaction solution was rotary evaporated to dryness, and 50 mL of dichloromethane and 50 mL of water were added in sequence, and after shaking well, the layers were separated and the organic dichloromethane layer was taken. After rotary evaporation to dryness, 16.6 g of thioether compound was obtained, with a yield of 96.8%. [M+H + ]=344. 1 H NMR(400MHz,DMSO)δ12.61(s,1H),8.23(d,J=5.6Hz,1H),7.46(d,J=51.5Hz,2H),7.12(dd,J=6.0,3.1Hz ,2H),6.95(d,J=5.7Hz,1H),4.69(s,2H),4.10(t,J=6.2Hz,2H),3.49(t,J=6.2Hz,2H),3.25(d ,J=0.5Hz,3H),2.21(s,3H),1.99(dd,J=12.4,6.2Hz,2H).
[0036] 1.2 Synthesis...
Embodiment 2
[0039] 2.1 Oxide C——2-[[[4-(3-methoxypropoxy)-3-methyl-1-oxopyridin-2-yl]methyl]sulfonyl]benzimidazole—— Synthesis
[0040] Under the condition of an acetone dry ice bath, add 50 mL of dichloromethane, 5.0 g (0.015 mol) of the thioether compound prepared in step 1 and 15.5 g (0.09 mol) of 3-chlorobenzoic acid into the three-necked flask. Stir at room temperature for 24h. Add excess saturated aqueous sodium bicarbonate solution, shake well, let stand to separate the layers, take the dichloromethane layer, and spin dry. After purification by HPLC chromatography, 3.9 g of oxide C was obtained with a yield of 66%. [M+H + ]=392.
[0041] 2.2 Oxide C——2-[[[4-(3-methoxypropoxy)-3-methyl-1-oxopyridin-2-yl]methyl]sulfonyl]benzimidazole—— Synthesis
[0042] Oxide C was prepared as above, except that the solvent used was 50 mL of 1,4-dioxane. 3.8 g of oxide C were obtained with a yield of 64%.
Embodiment 3
[0044] 3.1 Rabeprazole related product D——2-[[[4-(3-methoxypropoxy)-3-methyl-2-pyridyl]methyl]sulfonyl]benzimidazole—— synthesis
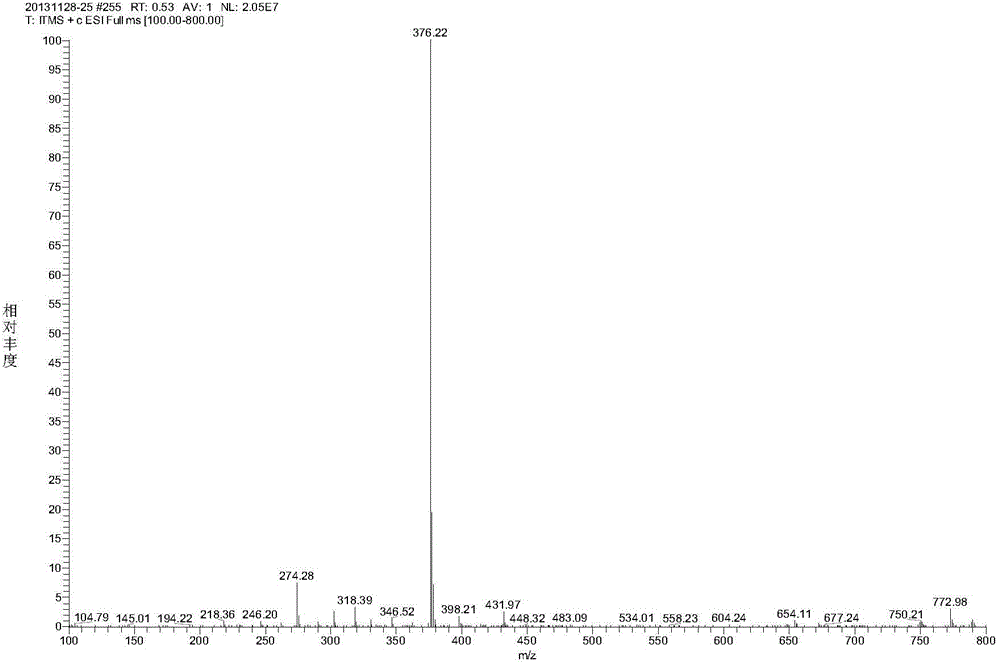
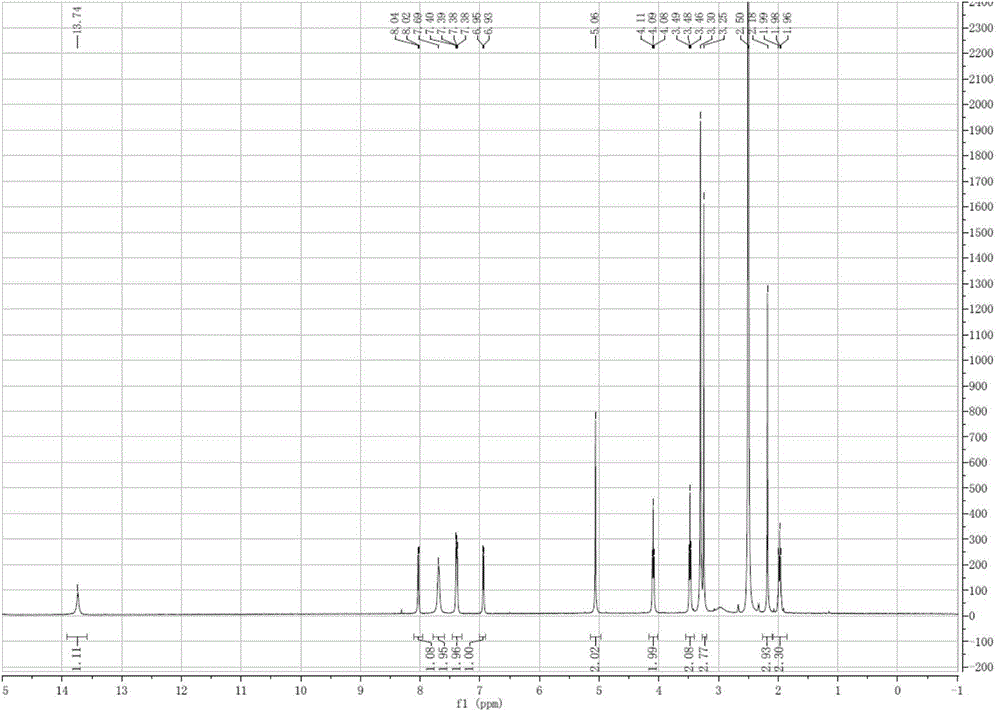
[0045] Add 3.92g (0.01mol) of oxide C prepared in Example 2, 6.5g of reduced zinc powder, 6g of glacial acetic acid and 50mL of water into the three-necked flask, and stir at 95°C for 24h to obtain rabeprazole Related material D crude. After purification by HPLC chromatography, 3.56 g of rabeprazole related product D (purity: 99.5%) can be obtained with a yield of 94.9%. [M+H + ]=376. 1H NMR(400MHz,DMSO)δ13.74(s,1H),8.03(d,J=5.5Hz,1H),7.69(s,2H),7.39(dd,J=6.1,3.1Hz,2H),6.94 (d,J=5.6Hz,1H),5.06(s,2H),4.09(t,J=6.1Hz,2H),3.48(t,J=6.2Hz,2H),3.25(s,3H),2.18 (s, 2H), 2.02-1.93 (m, 2H).
[0046] 3.2 Rabeprazole related substance D——2-[[[4-(3-methoxypropoxy)-3-methyl-2-pyridyl]methyl]sulfonyl]benzimidazole—— synthesis
[0047] Rabeprazole related product D was prepared as above, except that the reducing agent used was 6 g of reduced iron powder. 3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com