Metal super-hydrophobic surface and preparation method thereof
A super-hydrophobic surface and metal technology, which is applied in the field of metal super-hydrophobic surface and its preparation, can solve the problem of weak friction resistance of super-hydrophobic surface, and achieve the effect of simple and controllable electroplating process, low cost, and resistance to freezing water resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Polish the brass sheet with 400 mesh and 800 mesh sandpaper, rinse it with pure water, dry it, and use it as the cathode, and use the same size brass sheet as the anode. -1 Nickel sulfate, 50g?l -1 Nickel chloride hexahydrate and 45g?l -1 Electroplating is carried out in the Watts electroplating solution of boric acid, and the electroplating current density is 0.5A?cm -2 , the electroplating temperature is 50°C, and the electroplating time is 60 min. After the electroplating is completed, the workpiece is taken out, washed with water, and dried.
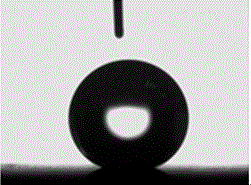
[0036] Place the electroplated workpiece together with 5% heptadecafluorodecyltriethoxysilane ethanol solution in an oven, heat treatment at 100°C for 60 min, take out the sample after heat treatment, and test its hydrophobicity, corrosion resistance and friction resistance Performance, the results are shown in Table 1, and its contact angle diagram is shown in figure 1 shown.
[0037]
Embodiment 2
[0039] Polish the brass sheet with 400 mesh and 800 mesh sandpaper, rinse it with pure water, dry it, and use it as the cathode, and use the same size brass sheet as the anode. -1 Nickel sulfate, 50g?l -1 Nickel chloride hexahydrate and 45g?l -1 Electroplating is carried out in the Watts electroplating solution of boric acid, and the electroplating current density is 0.3A?cm -2 , the electroplating temperature is 30°C, and the electroplating time is 30 min. After the electroplating is completed, the workpiece is taken out, washed with water, and dried.
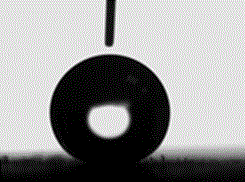
[0040] Place the electroplated workpiece together with 1% heptadecafluorodecyltriethoxysilane ethanol solution in an oven, and heat-treat at 120°C for 30 minutes. After heat-treatment, take out the sample and test its hydrophobicity, corrosion resistance and friction resistance Performance, the results are shown in Table 1, and its contact angle diagram is shown in figure 2 shown.
Embodiment 3
[0042] Polish the aluminum sheet with 400 mesh and 800 mesh sandpaper, rinse it with pure water, dry it, and use it as the cathode, and use the same size brass sheet as the anode. -1 Nickel sulfate, 50g?l -1 Nickel chloride hexahydrate and 45g?l -1 Electroplating was carried out in the Watts electroplating solution of boric acid, and the electroplating current density was 0.4 A?cm -2 , the electroplating temperature is 50°C, and the electroplating time is 40 min. After the electroplating is completed, the workpiece is taken out, washed with water, and dried.
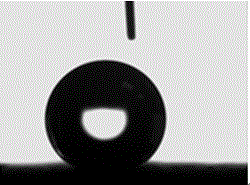
[0043] Place the electroplated workpiece together with the ethanol solution of heptadecafluorodecyltriethoxysilane with a concentration of 10% by volume in an oven, heat treatment at 150°C for 10 minutes, take out the sample after heat treatment, and test its hydrophobicity, corrosion resistance and friction resistance Performance, the results are shown in Table 1, and its contact angle diagram is shown in image 3 s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| contact angle | aaaaa | aaaaa |
| angle | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com