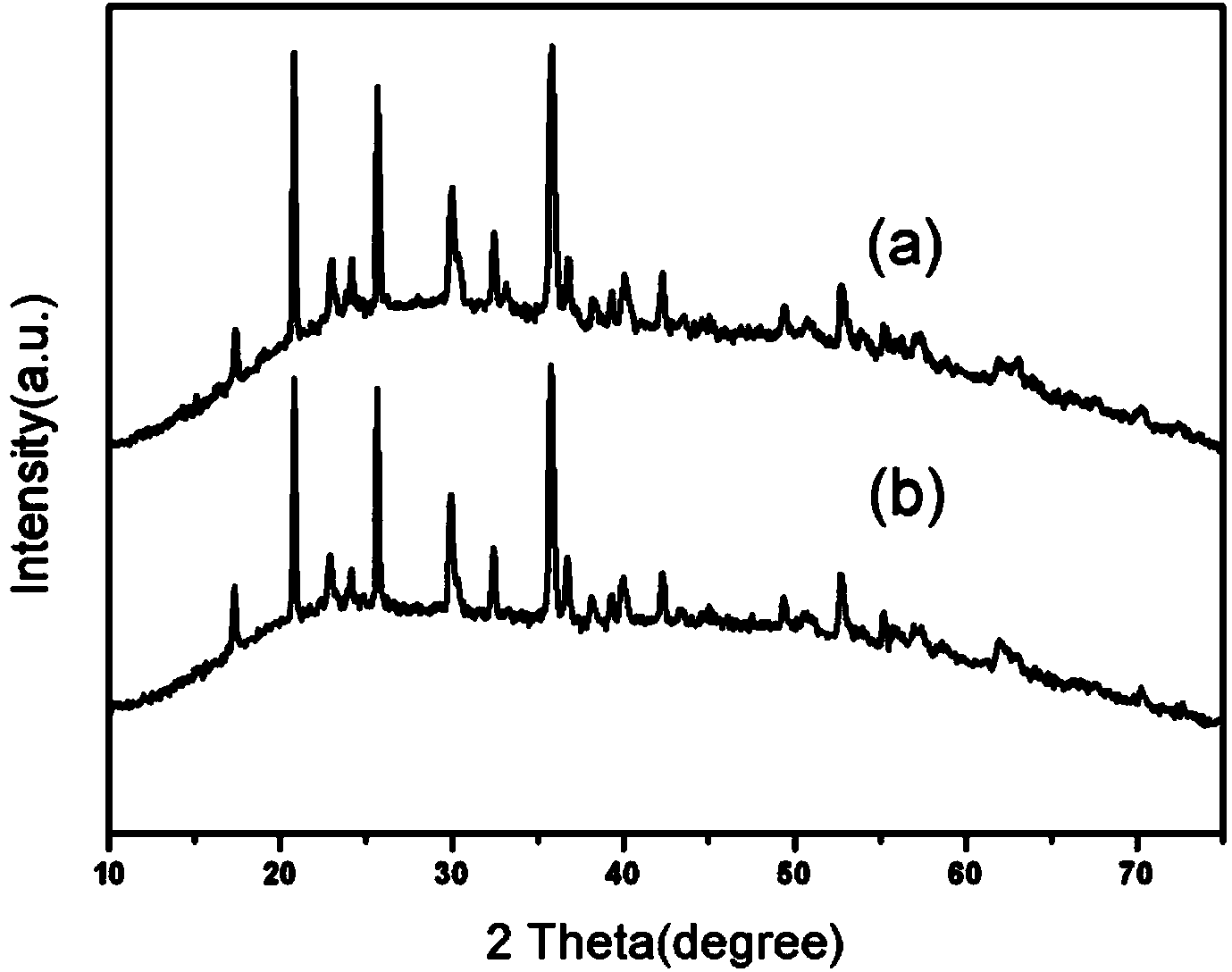
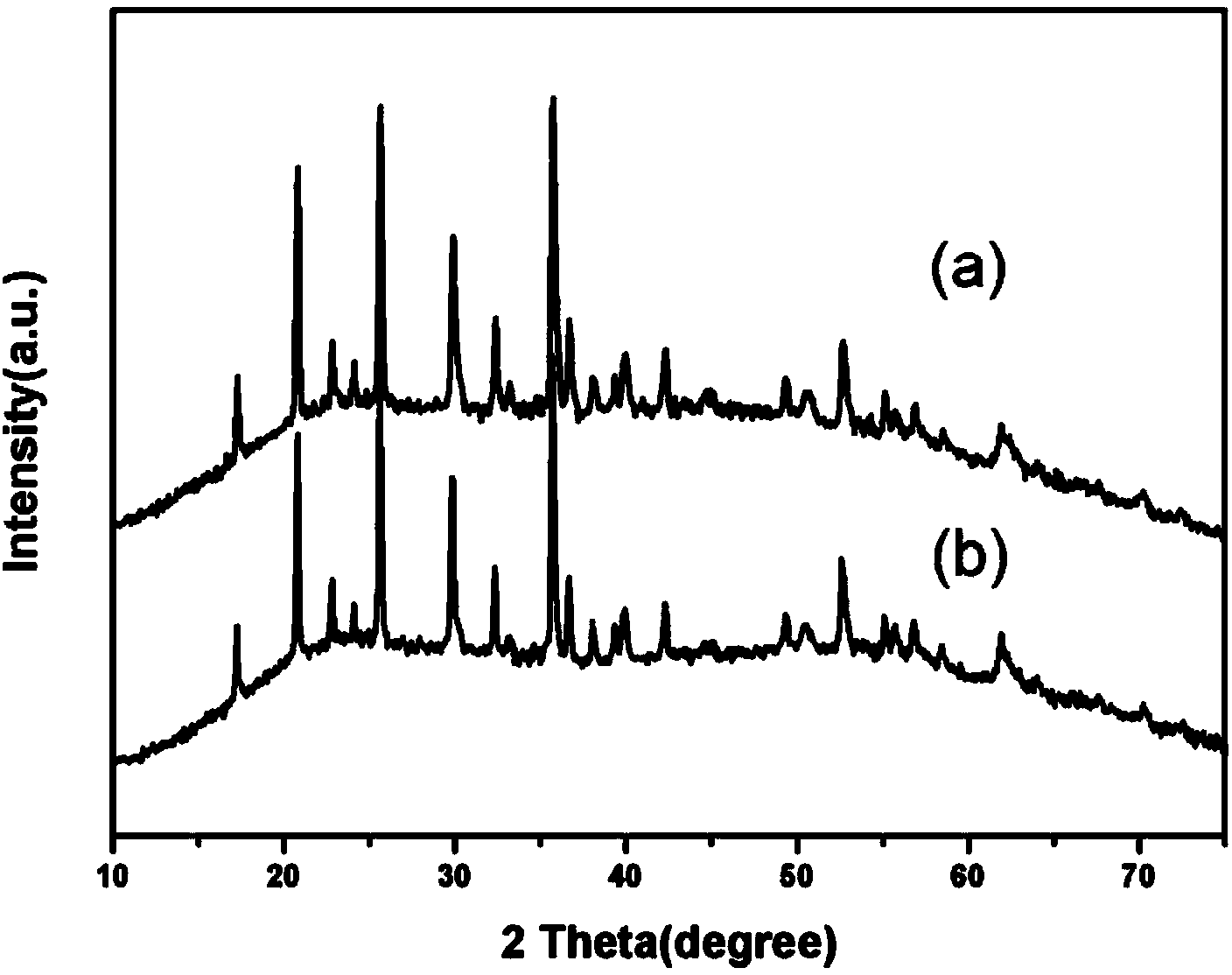
Preparation method of carbon-coated lithium iron phosphate with grade structure
A hierarchical structure, lithium iron phosphate technology, applied in structural parts, chemical instruments and methods, phosphorus compounds, etc., can solve problems affecting the chemical and electrochemical properties of products, achieve good development prospects, improve purity, and have a wide range of raw material sources Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Take LiAc·2H 2 O, Fe(NO 3 ) 3 9H 2 O, H 3 PO 4 As raw material, 0.01mol LiAc·2H 2 O and 0.01molFe(NO 3 ) 3 9H 2 O was dissolved in 70ml of diethylene glycol (DEG), and after stirring evenly, 0.01mol H 3 PO 4 , stirred for 30min, added 3ml of ethylenediamine (EN) dropwise, stirred for 30min, transferred the stirred solution into a 100ml hydrothermal kettle, heated it at 220°C for 24h, then cooled to room temperature, and deionized the reaction solution After multiple washings with water and ethanol, they were oven dried at 100°C. Dry the spare LiFePO 4 Powder and sucrose (the amount of sucrose added to make the carbon content 4wt%) is mixed and dissolved in deionized water, stirred with a glass rod until dry, and then dried in a vacuum oven at 100°C after stirring, and the dried powder is reduced Atmosphere (5vol.%H 2 and 95vol.% Ar) to 700°C and calcined for 10h. Naturally cooled to room temperature with the furnace to obtain the carbon-coated lithium iron ...
Embodiment 2
[0038] Take LiAc·2H 2 O, Fe(NO 3 ) 3 9H 2 O, H 3 PO 4 As raw material, 0.0102mol LiAc·2H 2 O and 0.01molFe(NO 3 ) 3 9H 2O was dissolved in 35ml of diethylene glycol (DEG), and after stirring evenly, 0.01mol H 3 PO 4 , stirred for 30min, added 3ml of ethylenediamine (EN) dropwise, stirred for 30min, transferred the stirred solution into a 100ml hydrothermal kettle, heated it at 220°C for 24h, then cooled to room temperature, and deionized the reaction solution After multiple washings with water and ethanol, they were oven dried at 100°C. Dry the spare LiFePO 4 Powder and sucrose (the amount of sucrose added to make the carbon content 4wt%) is mixed and dissolved in deionized water, stirred with a glass rod until dry, and then dried in a vacuum oven at 100°C after stirring, and the dried powder is reduced Atmosphere (5vol.%H 2 and 95vol.% Ar) to 700°C and calcined for 10h. Naturally cooled to room temperature with the furnace to obtain the carbon-coated lithium iron...
Embodiment 3
[0041] Take LiAc·2H 2 O, FeCl 3 、H 3 PO 4 As raw material, 0.01mol LiAc·2H 2 O and 0.01mol FeCl 3 Dissolve in 70ml ethylene glycol (EG), stir evenly, add 0.01mol H 3 PO 4 , stirred for 30min, added 4ml of ethylenediamine (EN) dropwise, stirred for 30min, transferred the stirred solution into a 100ml hydrothermal kettle, heated it at 220°C for 36h, then cooled to room temperature, and deionized the reaction solution After multiple washings with water and ethanol, they were oven dried at 100°C. Dry the spare LiFePO 4 Powder and sucrose (the amount of sucrose added to make the carbon content 4wt%) is mixed and dissolved in deionized water, stirred with a glass rod until dry, and then dried in a vacuum oven at 100°C after stirring, and the dried powder is reduced Atmosphere (5vol.%H 2 and 95vol.% Ar) to 750°C and calcined for 10h. Naturally cooled to room temperature with the furnace to obtain the carbon-coated lithium iron phosphate cathode material LiFePO 4 / C.
[004...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com