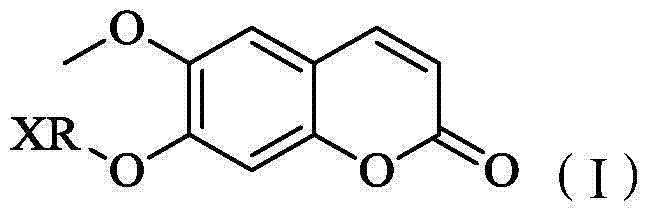
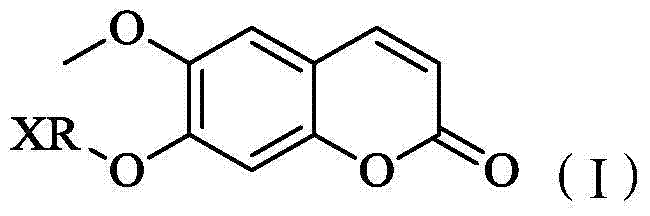
Scopoletin derivative as well as preparation method and application thereof
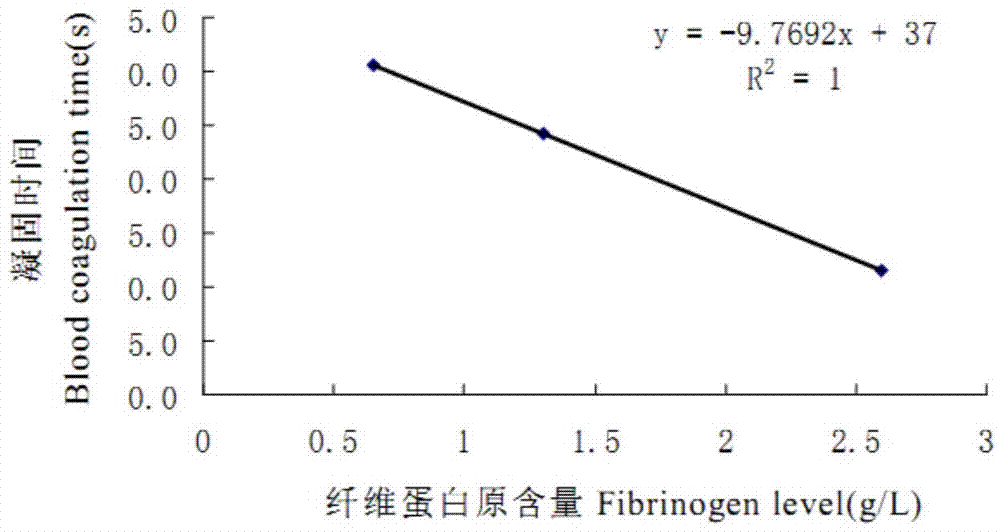
A technology of scopolide and derivatives is applied in the field of scopolide derivatives and their preparation, which can solve the problems such as inability to take orally and achieve the effects of small dosage, easy control and prolonging in vitro coagulation time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0042] The preparation method of above-mentioned scopoletin derivative or pharmaceutically acceptable salt, comprises the following steps:
[0043] Scopoletin, XRX and anhydrous potassium carbonate were dissolved in an organic solvent, stirred and reacted at 50° C. for 12 hours to obtain a scopoletin derivative. The molar ratio of scopoletin, XRX and anhydrous potassium carbonate is 1:1:1-5. Wherein, the organic solvent is used to dissolve scopoletin and XRX, and in this embodiment, the organic solvent is acetonitrile. In one embodiment, after the reaction is completed, there is a post-processing step. The specific operation is: cooling the mixed solution to room temperature, filtering, recovering the filtrate under reduced pressure, dissolving the residue with ethyl acetate, and adding an appropriate amount of KOH under stirring to react After 2 hours to remove unreacted raw materials, filter, and recover the filtrate under reduced pressure to obtain the target mixed product...
Embodiment 1
[0050] The preparation of embodiment 1 scopoletin
[0051] The synthetic route is as follows:
[0052]
[0053] Add 2,4,5-trimethoxybenzaldehyde a (24.53 g, 0.125 mol) dropwise to a stirred suspension of aluminum trichloride (67 g, 0.5 mol) in dry dichloromethane (350 mL) at room temperature The dichloromethane (125 mL) solution was dried. After the dropwise addition, the stirring reaction was continued at room temperature for 4 hours, and then another dispersion of aluminum trichloride (67 g, 0.5 mol) in dry dichloromethane (150 mL) was added. The mixed solution was stirred and reacted for 19 hours, then poured into 1 kg of ice added with 45 mL of concentrated hydrochloric acid, and the organic layer was separated, and the aqueous layer was washed twice with 200 mL of dichloromethane respectively, and the organic layers were combined, filtered through silica gel, evaporated to dryness, and weighed in ethyl acetate. The crystallized compound b was 2,4-dihydroxy-5-methoxyb...
Embodiment 2
[0058] The preparation of embodiment 2 scopoletin derivatives
[0059] The synthetic route is as follows:
[0060]
[0061] Take scopoletin 576mg (3mM), XRX 3mM and an appropriate amount of anhydrous potassium carbonate, dissolve in 9mL acetonitrile, stir and react at 20-50°C (under reflux) for 6-24h, cool the mixture to room temperature, filter, and depressurize the filtrate Recovery, the residue was dissolved with 3 mL of ethyl acetate, and an appropriate amount of KOH was added to react for 2 h under stirring to remove unreacted raw materials, filtered, and the filtrate was recovered under reduced pressure to obtain the target mixed product. The mixture mainly had two spots,
[0062] Use 200-300 mesh silica gel for column chromatography separation, respectively, petroleum ether-ethyl acetate gradient elution, LH-20 purification, TLC detection, after 1 H-NMR confirmed that it was the corresponding scopoletin derivative. Compound number, XRX and the yield of correspondin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com