Star-like visible light initiator having conjugated structure and containing benzophenone fragments as well as synthesis and application of visible light initiator
A benzophenone and conjugated structure technology is applied in the field of star-shaped visible light initiators, which can solve the problems of short absorption wavelength and low initiation efficiency, and achieve the effects of simple synthesis method, convenient source of raw materials and easy-to-obtain raw material sources.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Synthesis of 4-benzoyl-styryl-4',4''-di(phenyl)triphenylamine
[0040] The synthesis proceeds in two steps:
[0041] (1) Synthesis of 4-benzoyl-styryl-4’,4’’-dibromotriphenylamine
[0042]4-benzoyl-benzyl-diethyl phosphate (7.54mmol, 2.51g) and 4-formyl-4',4''-dibromotriphenylamine (5.8mmol, 2.5g) were dissolved in 50ml of dry tetrahydrofuran In , sodium methoxide (17.4mmol, 0.94g) was used as a basic catalyst and reacted at room temperature for 24 hours. After tetrahydrofuran was removed under reduced pressure, it was extracted with dichloromethane, and the organic layer was collected and dried over anhydrous sodium sulfate, and the solvent was removed under reduced pressure. After column separation, 4-benzoyl-styryl-4',4''-dibromotriphenylamine was obtained, and recrystallized in benzene to obtain a yellow solid with a yield of 60%.
[0043] (2) Synthesis of 4-benzoyl-styryl-4’,4’’-di(phenyl)triphenylamine
[0044] 4-benzoyl-styryl-4',4''-dibromotriphenylamine (3....
Embodiment 2
[0048] Synthesis of 4-benzoyl-styryl-4',4''-di(methyl-phenyl)triphenylamine
[0049] 4-Benzoyl-styryl-4',4''-dibromotriphenylamine (3.77mmol, 2.3g) and p-tolylboronic acid (4.524mmol, 0.615g) were dissolved in 100ml of dry tetrahydrofuran, carbonic acid Potassium (18.85mmol, 2.6g) was used as a basic catalyst, and was refluxed for 24 hours under the protection of argon. After tetrahydrofuran was removed under reduced pressure, it was extracted with dichloromethane, and the organic layer was collected and dried over anhydrous sodium sulfate, and the solvent was removed under reduced pressure. After column separation, 4-benzoyl-styryl-4',4''-di(methyl-phenyl)triphenylamine was obtained, and recrystallized in benzene to obtain an orange solid with a yield of 73%.
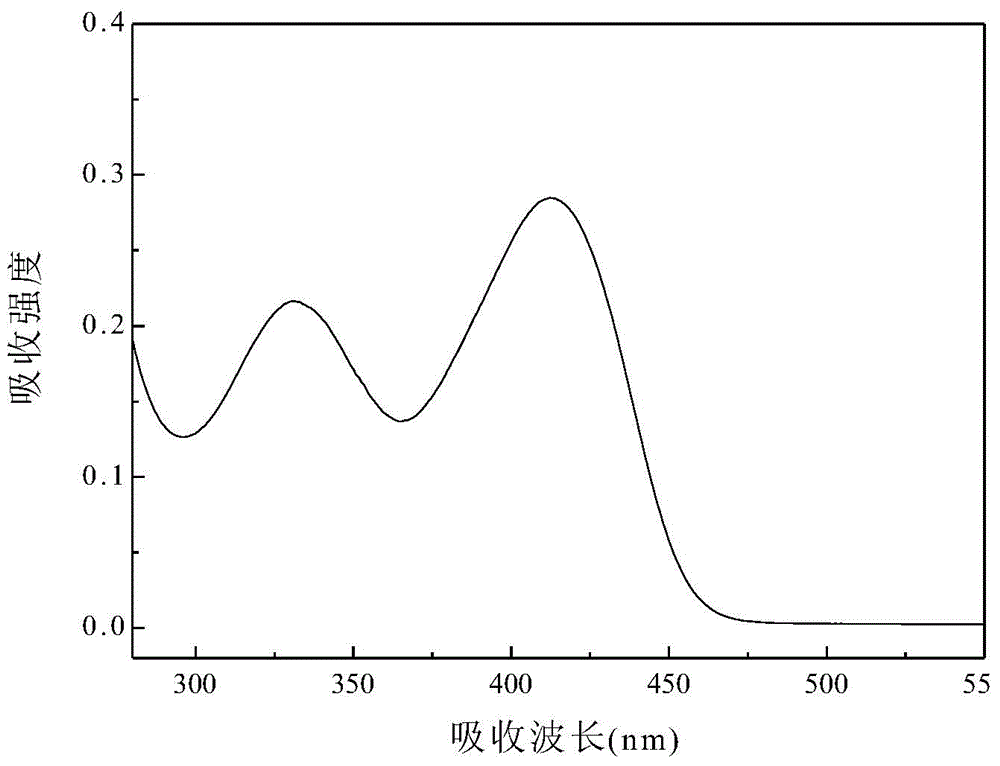
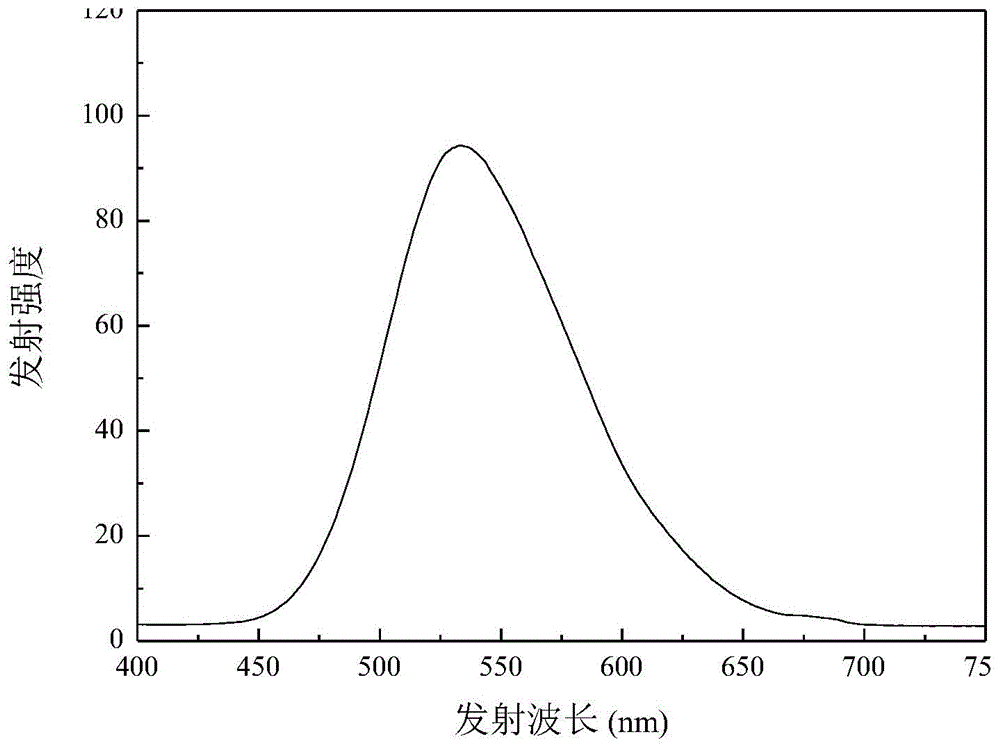
[0050] The prepared 4-benzoyl-styryl-4',4''-bis(methyl-phenyl)triphenylamine was formulated into 1×10 in dichloromethane -5 mol / L solution, measure its ultraviolet absorption spectrum. Such as image 3 As shown, th...
Embodiment 3
[0053] Synthesis of 4-benzoyl-styryl-4',4''-bis(methoxy-phenyl)triphenylamine
[0054] 4-benzoyl-styryl-4',4''-dibromotriphenylamine (3.77mmol, 2.3g) and p-methoxyphenylboronic acid (4.524mmol, 0.688g) were dissolved in 100ml of dry tetrahydrofuran, Potassium carbonate (18.85mmol, 2.6g) was used as a basic catalyst, and was refluxed for 24 hours under the protection of argon. After tetrahydrofuran was removed under reduced pressure, it was extracted with dichloromethane, and the organic layer was collected and dried over anhydrous sodium sulfate, and the solvent was removed under reduced pressure. After column separation, 4-benzoyl-styryl-4',4''-di(methoxy-phenyl)triphenylamine was obtained, which was recrystallized in benzene to obtain an orange solid with a yield of 75%.
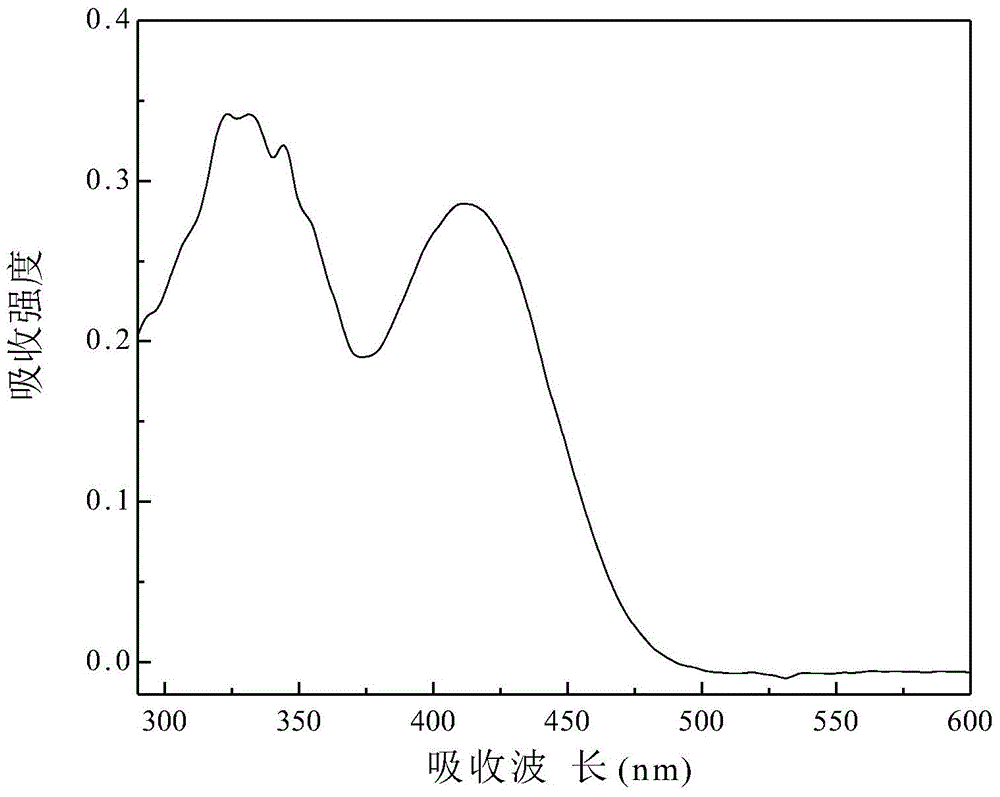
[0055] The prepared 4-benzoyl-styryl-4',4''-bis(methoxy-phenyl)triphenylamine was prepared in dichloromethane to 1×10 -5 mol / L solution, measure its ultraviolet absorption spectrum. Such as Figure 5 A...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com