A kind of manufacturing process of lithium polysulfide required for lithium-sulfur battery
A technology for lithium polysulfide and lithium sulfur batteries, which is applied in lithium batteries, alkali metal sulfides/polysulfides, sulfur compounds, etc., can solve the problems of increasing the use cost of lithium sulfur batteries, poor stability of vulcanized polymers, and high difficulty.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
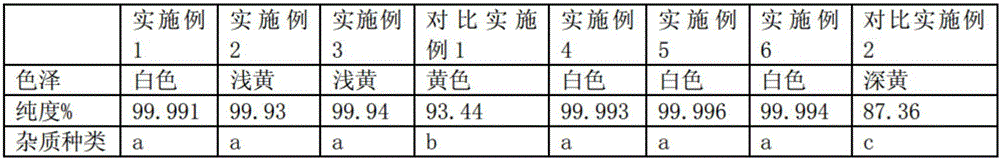
Examples
Embodiment 1
[0031] In the reaction kettle for synthesizing lithium polysulfide, after the replacement of inert gas, under the condition that the flow rate of continuously feeding nitrogen is 360ml / min, add 4.4mol of NMP solvent, 1.0mol of sodium hydrosulfide, and 1.03mol of sodium hydroxide and 0.3mol of deionized oxygen-free water, then heated the temperature of the reaction system from room temperature to 66°C, and kept it at this temperature for 33min, then added 1.001mol of lithium chloride, and continued to increase the temperature of the reaction system to 180°C, And under this temperature and condition, make the reaction system keep warm for 33min, when fine white crystals are precipitated in the reaction system, remove the water content in the reaction system by evaporation, stop heating, and filter while hot to remove the sodium chloride generated , the filtrate was returned to the reaction system, and the air in it was replaced with nitrogen, then 4.0mol of sublimed sulfur was ad...
Embodiment 2
[0034] In the reaction kettle for synthesizing lithium polysulfide, after the replacement of inert gas, under the condition that the flow rate of nitrogen gas is continuously fed into 480ml / min, 5.4mol of NMP solvent, 1.0mol of sodium hydrosulfide, and 1.03mol of sodium hydroxide are added and 0.3mol deionized oxygen-free water, then heat the temperature of the reaction system from room temperature to 68°C, and keep it at this temperature for 18 minutes, then add 1.001mol lithium chloride, and continue to increase the temperature of the reaction system to 180°C, And under this temperature and condition, make the reaction system keep warm for 18min, when fine white crystals are precipitated in the reaction system, remove the water content in the reaction system by evaporation, stop heating, and filter while hot to remove the sodium chloride generated , the filtrate was returned to the reaction system, and the air in it was replaced with nitrogen, then 6.0mol of sublimed sulfur w...
Embodiment 3
[0037] In the reaction kettle for synthesizing lithium polysulfide, after the replacement of inert gas, under the condition that the flow rate of nitrogen gas is continuously fed into 380ml / min, add 4.6mol of NMP solvent, 1.0mol of sodium hydrosulfide, and 1.03mol of sodium hydroxide and 0.3mol deionized oxygen-free water, then heat the temperature of the reaction system from room temperature to 67°C, and keep it at this temperature for 21 minutes, then add 1.001mol lithium chloride, and continue to increase the temperature of the reaction system to 163°C, And under this temperature and condition, make the reaction system keep warm for 19min, when fine white crystals are separated out in the reaction system, the water content in the reaction system is evaporated and removed at the same time, and stop heating, filter while hot to remove the sodium chloride that generates , the filtrate was returned to the reaction system, and the air in it was replaced with nitrogen, then 4.5mol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com