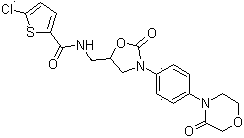
Method for preparing rivaroxaban
A technology for rivaroxaban and intermediates, applied in the field of preparation of rivaroxaban, can solve the problems of long reaction steps, low route yield, unfavorable large-scale industrial production, etc., achieve low cost, cheap raw materials, and simple operation easy to control effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
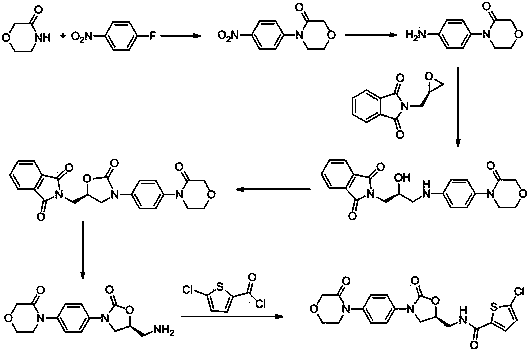
[0023] Synthesis of intermediate Ⅰ: 19g 4-(4-aminophenyl)-3-morpholinone and 20g 2-[(2S)-2-oxiranyl-methyl]-1H-isoindole- 1,3(2H)-dione was placed in 180ml of isopropanol and 100ml of water, stirred, heated to 50°C, 19.4g of CDI was dissolved in 50mL of tetrahydrofuran, slowly added dropwise to the above reaction solution, and then heated to reflux for reaction, The reaction was monitored by TLC; after the reaction was completed, when cooled to room temperature, tetrahydrofuran and isopropanol were evaporated under reduced pressure, then heated to 60°C, slowly added 100mL of ethanol dropwise, stirred, cooled to 20°C, suction filtered, and dried to obtain 41g of crude solid , put the solid crude product in 240mL of isopropanol, heat to dissolve, cool down to 15°C to crystallize, filter with suction, and dry to obtain 35.4g of white solid product, yield: 85%;
[0024] Synthesis of Intermediate II: Put 35g of Intermediate I in 280ml of acetonitrile, stir, heat up to 50°C, add 20g...
Embodiment 2
[0027] Synthesis of intermediate Ⅰ: 23g 4-(4-aminophenyl)-3-morpholinone and 29g 2-[(2S)-2-oxiranyl-methyl]-1H-isoindole- 1,3(2H)-diketone was placed in 240ml of isopropanol and 120ml of water, stirred, heated to 50°C, 24.7g of DCC was dissolved in 80mL of tetrahydrofuran, slowly added dropwise to the above reaction solution, and then heated to reflux for reaction, The reaction was monitored by TLC; after the reaction was completed, when cooled to room temperature, tetrahydrofuran and isopropanol were evaporated under reduced pressure, then heated to 60°C, slowly added 150mL of ethanol dropwise, stirred, cooled to 20°C, suction filtered, and dried to obtain 48g solid crude product , put the solid crude product in 280mL of isopropanol, heat to dissolve, cool down to 15°C to crystallize, filter with suction, and dry to obtain 43.5g of white solid product, yield: 87%;
[0028] Synthesis of Intermediate II: Put 40g of Intermediate I in 350ml of tetrahydrofuran, stir, heat up to 45...
Embodiment 3
[0031] Synthesis of intermediate Ⅰ: 28g 4-(4-aminophenyl)-3-morpholinone and 37g 2-[(2S)-2-oxiranyl-methyl]-1H-isoindole- 1,3(2H)-dione was placed in 300ml of isopropyl ether and 150ml of water, stirred, heated to 50°C, 39.1g of CDI was dissolved in 100mL of tetrahydrofuran, slowly added dropwise to the above reaction solution, and then heated to reflux for reaction , TLC monitors the reaction, the reaction is completed, cooled to room temperature, evaporated under reduced pressure to remove tetrahydrofuran and isopropyl ether, then heated to 60 degrees Celsius, slowly added 200mL of ethanol dropwise, stirred, cooled to 20 degrees Celsius, suction filtered, and dried to obtain 56g of solid Crude product, put the solid crude product in 350mL of isopropanol, heat to dissolve, cool down to 15°C to crystallize, filter with suction, and dry to obtain 51g of white solid product, yield: 85%;
[0032] Synthesis of Intermediate II: Put 48g of Intermediate I in 400ml of ethanol, stir, h...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com