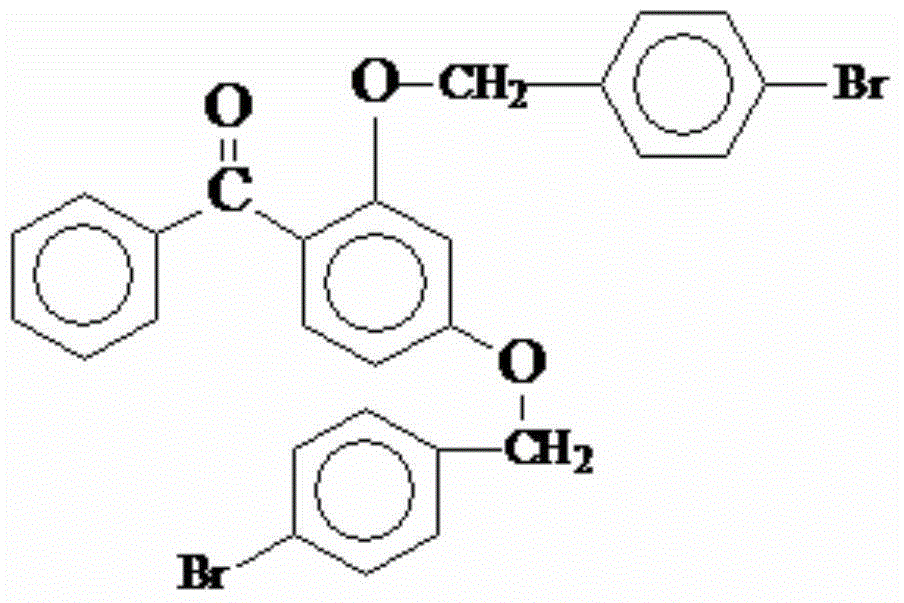
Preparation method and application of antioxidant 2-4-p-bromobenzyl benzophenone
A bromobenzyl benzophenone and antioxidant technology, applied in the field of antioxidant preparation, can solve the problems of complex antioxidant preparation process, environmental pollution, equipment corrosion, etc., achieve remarkable effect, expand specific surface area, and improve stability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] One, coated antioxidant 2,4-p-bromobenzyl benzophenone, prepared through the following steps:
[0026] (a).Chemical components: 50 parts of 2,4 dihydroxybenzophenone, 100 parts of p-bromobenzyl bromide, 5 parts of solid alkali carrier dilute metal element catalyst, KCO 3 20 parts, 0.1 part of cerium trichloride, 1 part of benzyltriethylammonium chloride;
[0027] (b). 2,4 dihydroxy benzophenone, p-bromobenzyl bromide, solid alkali carrier dilute metal element catalyst, KCO 3 , cerium trichloride, and benzyltriethylammonium chloride were placed in a four-necked flask, fed with nitrogen, and reacted at reflux at 100°C for 6 hours, filtered the solid matter while it was hot, and then separated the reacted aqueous phase and organic phase Separate, then extract the organic phase with 100 parts of 0.1% sodium chloride aqueous solution, extract 3 times, and then extract the organic phase with 100 parts of distilled water, distill under reduced pressure, and then cool to obtai...
Embodiment 2
[0035] One, coated stone paper antioxidant 2,4-p-bromobenzyl benzophenone, prepared by the following steps:
[0036] (a). Chemical components: 80 parts of 2,4 dihydroxybenzophenone, 160 parts of p-bromobenzyl bromide, 10 parts of solid base carrier dilute metal element catalyst, KCO 3 30 parts, 0.3 parts of cerium trichloride, 1.5 parts of benzyltriethylammonium chloride;
[0037] (b). 2,4 dihydroxy benzophenone, p-bromobenzyl bromide, solid alkali carrier dilute metal element catalyst, KCO 3 , cerium trichloride, and benzyltriethylammonium chloride were placed in a four-necked flask, fed with nitrogen, and reacted at reflux for 3 hours at 115°C, filtered the solid matter while it was hot, and then separated the aqueous phase and the organic phase after the reaction , then use 100 parts of 0.1% sodium chloride aqueous solution to extract the organic phase, extract 3 times, and then use 100 parts of distilled water to extract the organic phase, then distill under reduced press...
Embodiment 3
[0045] One, coated antioxidant 2,4-p-bromobenzyl benzophenone, prepared through the following steps:
[0046] (a). Chemical components: 65 parts of 2,4 dihydroxybenzophenone, 130 parts of p-bromobenzyl bromide, 7.5 parts of solid alkali carrier dilute metal element catalyst, KCO 3 25 parts, 0.2 parts of cerium trichloride, 2 parts of benzyltriethylammonium chloride;
[0047] (b). 2,4 dihydroxy benzophenone, p-bromobenzyl bromide, solid alkali carrier dilute metal element catalyst, KCO 3 , cerium trichloride, and benzyltriethylammonium chloride were placed in a four-necked flask, fed with nitrogen, and reacted at reflux at 115°C for 4.5 hours, filtered the solid matter while it was hot, and then separated the aqueous phase and the organic phase after the reaction , then use 100 parts of 0.1% sodium chloride aqueous solution to extract the organic phase, extract 3 times, and then use 100 parts of distilled water to extract the organic phase, then distill under reduced pressure,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com