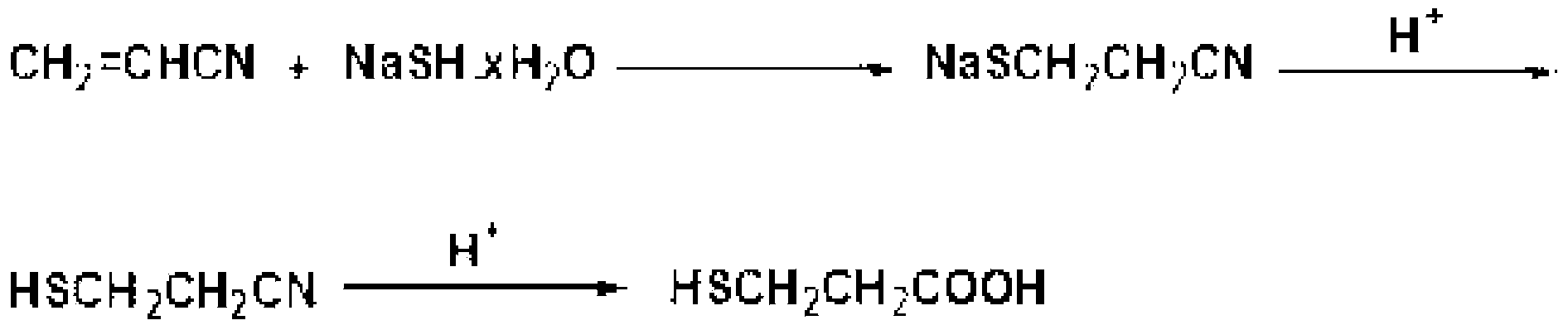Method for producing 3-mercaptopropionic acid, and carbonic acid ester composition having mercapto group using same, and method for producing thiourethane-based optical materials
A technology of mercaptopropionic acid and its manufacturing method, which is applied in optics, organic chemistry, optical components, etc., can solve problems such as complex manufacturing process, difficult manufacturing, complex reaction steps, etc., and achieve simple manufacturing process, excellent yield, and excellent hue Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 1
[0070] 3-Mercaptopropionic acid (MPA-1)
[0071] After installing a stirrer, a thermometer, and a condenser in a 1-liter 4-zone flask, put NaSH.xH 2 O (70%, 162.00g, 2.00 moles), add 160g of water, and stir for 30 minutes at 30°C to dissolve completely. Next, maintaining a temperature of 35° C., acrylonitrile (106.12 g, 2.00 mol) was slowly added dropwise. After heating and starting the reaction, all the acrylonitrile was charged, and then left at 60°C for 8 hours to obtain sodium 2-cyanoethanethiolate. The product was confirmed by GC analysis. The starting substance completely disappeared, and the product sodium 2-cyanoethanethiolate was neutralized to obtain 3-mercaptopropionitrile, which was confirmed by GC analysis. After the reaction was completed, the temperature was lowered to 10° C., and concentrated hydrochloric acid was slowly added dropwise while stirring for neutralization. After the dropwise addition, the stirring was stopped, the nitriles in the upper layer ...
Synthetic example 2
[0073] 3-Mercaptopropionic acid (MPA-2)
[0074] After installing a stirrer, a thermometer, and a condenser in a 1-liter 4-zone flask, put NaSH.xH 2 O (70%, 177.79g, 2.22 moles), add 160g of water, and stir for 30 minutes at 40°C to dissolve completely. Next, maintaining a temperature of 35° C., acrylonitrile (106.12 g, 2.00 mol) was slowly added dropwise. Heat up and start to react. After all the acrylonitrile is put in, place it at 50°C for 10 hours, stir at this temperature to obtain sodium 2-cyanoethanethiolate, and neutralize it to obtain 3-mercaptopropionitrile. 3-Mercaptopropionitrile was confirmed by GC analysis. When the reaction is finished, the temperature is lowered to 10°C, and neutralized by concentrated hydrochloric acid, and the generated 3-mercaptopropionitrile exists in the upper layer—the organic layer. The lower layer—the aqueous layer was removed, and concentrated hydrochloric acid was added to the upper layer 3-mercaptopropionitrile. React at 60°C f...
Synthetic example 3
[0081] Trimethylolpropane Tris(3-Mercaptopropionate) (TMPMP-2)
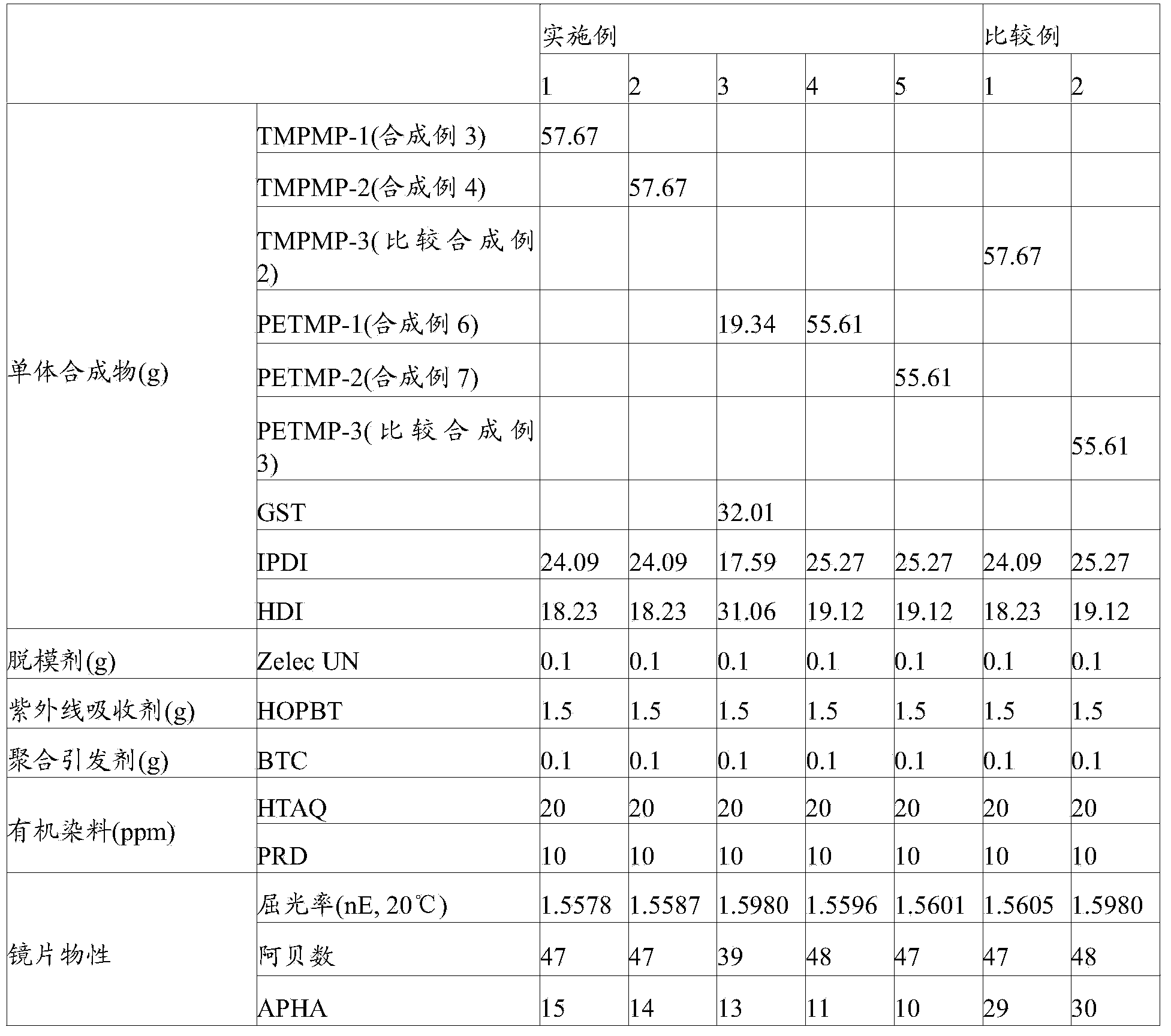
[0082]Install a stirrer, a thermometer and a Dean-stark device (Dean-stark) on a 1-liter 4-zone flask, add 1 mole (67.08 g) of trimethylol propane, and put the 3-mercaptopropionic acid (MPA) obtained in Synthesis Example 1 -1) 3 moles (318.42 g), 100 g of toluene was added as a solvent, and it was placed in an oil heating pot and heated. The oil temperature was 150°C. When the internal temperature was around 120°C, water began to be generated, and the reaction continued for 24 hours. After that, the occurrence of water was hardly observed, and the excess 3-mercaptopropionic acid was distilled under reduced pressure in the solvent and recovered. As a result of LC analysis, unreacted trimethylpropane did not appear, the purity of the product was 88%, and 195.28 g of the product was obtained. The resulting product has a refractive index (nE) of 1.518 and a hue of APHA14, and can be directly used as a polymeric ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Center thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com